Your Location:Home >Products >Organic Chemistry >1530-32-1
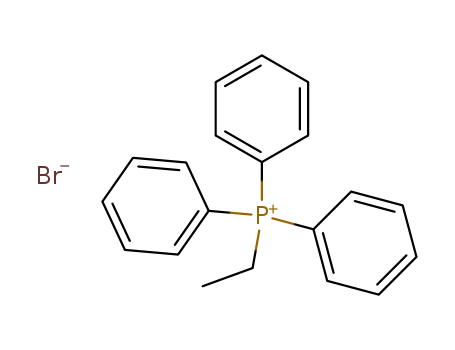

Product Details
|
Chemical Properties |
White to off-white crystalline powder |
|
Uses |
suzuki reaction |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Recrystallise it from H2O and dry it in high vacuum at 100o. IR has bands at 1449, 1431 and 997cm-1 . [Wittig & Wittenberg Justus Liebigs Ann Chem 606 1 1957, Bergmann & Dusza J Org Chem 23 1245 1958, Beilstein 16 IV 982.] |
InChI:InChI=1/C20H20BrP/c1-2-22(21,18-12-6-3-7-13-18,19-14-8-4-9-15-19)20-16-10-5-11-17-20/h3-17H,2H2,1H3
It was shown that it is possible to use ...
With the natural product (1R)-(-)-myrten...
The fluorinated phosphonium salt (Ph3P+C...
Oxidosqualene cyclases catalyze the tran...
When the coordinating isopropyl ether of...
A set of new substituted dienes were syn...
Progress toward a convergent approach fo...
The invention provides an isoxazoline de...
In this paper, we revealed a metal-free ...
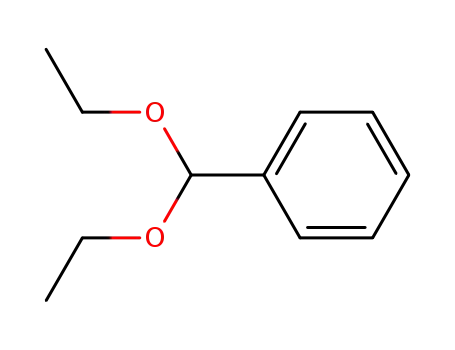
(diethoxymethyl)benzene

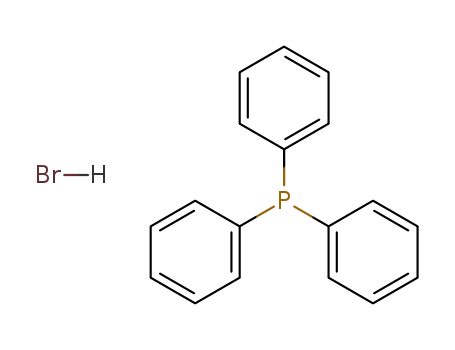
triphenylphosphine hydrobromide

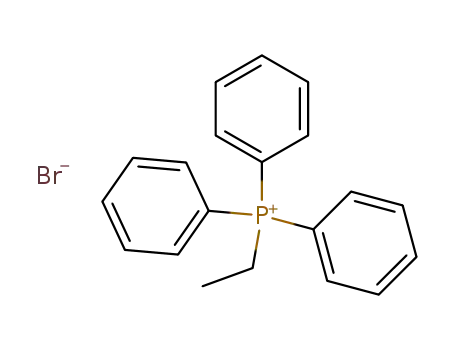
ethyltriphenylphosphonium bromide

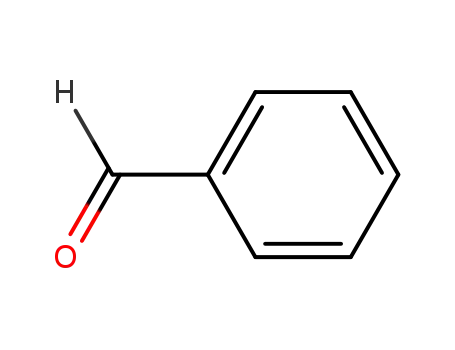
benzaldehyde
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
at 50 ℃;
for 0.0833333h;
Microwave irradiation;
Sealed tube;
|
73% 66% |
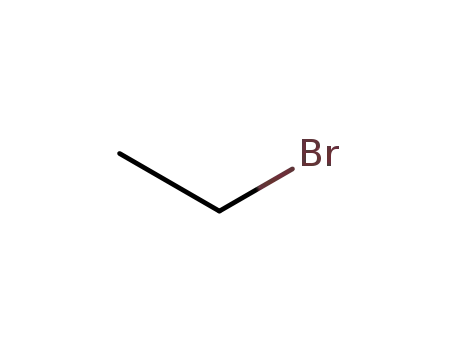
ethyl bromide

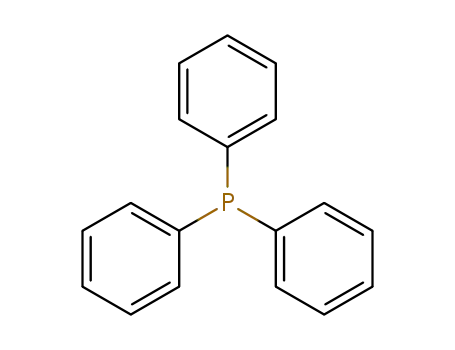
triphenylphosphine

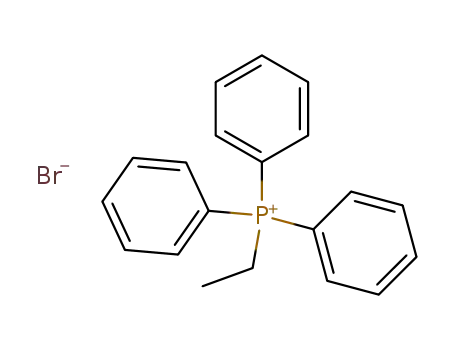
ethyltriphenylphosphonium bromide
| Conditions | Yield |
|---|---|
|
for 48h;
Heating;
|
98% |
|
In
acetonitrile;
Heating;
|
96.8% |
|
In
ethanol;
for 21h;
Heating;
|
94.2% |
|
at 100 ℃;
for 1h;
|
91% |
|
In
toluene;
for 10h;
Reflux;
|
91.8% |
|
In
benzene;
at 20 ℃;
for 4h;
|
90% |
|
In
benzene;
at 40 ℃;
for 48h;
|
89.2% |
|
for 120h;
Reflux;
Inert atmosphere;
|
87% |
|
In
toluene;
for 17h;
Inert atmosphere;
Schlenk technique;
Reflux;
|
84% |
|
In
toluene;
Reflux;
|
81% |
|
In
toluene;
at 70 ℃;
for 24h;
Inert atmosphere;
|
79% |
|
In
toluene;
at 115 ℃;
for 16h;
Inert atmosphere;
|
79% |
|
In
toluene;
at 75 ℃;
for 24h;
|
74% |
|
In
toluene;
at 100 ℃;
|
70.5% |
|
In
para-xylene;
at 110 ℃;
for 3h;
Inert atmosphere;
|
63% |
|
|
|
|
In
acetonitrile;
|
|
|
In
toluene;
at 90 ℃;
for 24h;
|
|
|
In
toluene;
for 24h;
Reflux;
|
|
|
In
toluene;
for 8h;
Reflux;
|
8.05 g |
|
for 62h;
Schlenk technique;
Inert atmosphere;
Reflux;
|
|
|
In
toluene;
for 16h;
Reflux;
|
|
|
In
acetonitrile;
Reflux;
|
|
|
In
toluene;
at 120 ℃;
for 24h;
|
|
|
In
5,5-dimethyl-1,3-cyclohexadiene;
at 130 ℃;
for 12h;
|
|
|
Reflux;
|

ethyl bromide

triphenylphosphine

(diethoxymethyl)benzene
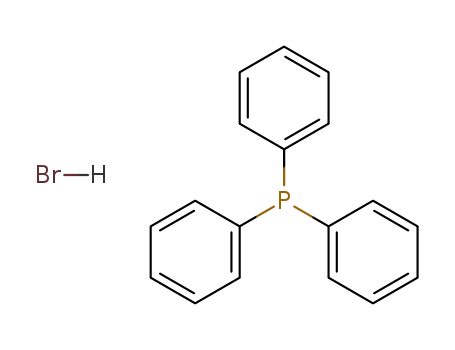
triphenylphosphine hydrobromide
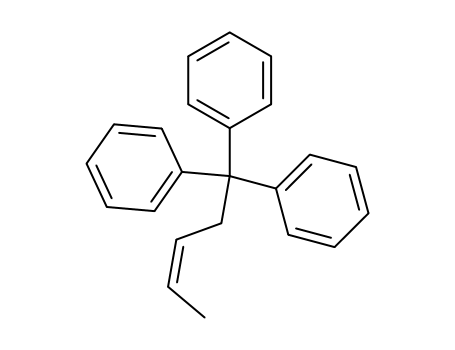
5,5,5-triphenyl-pent-2c-ene
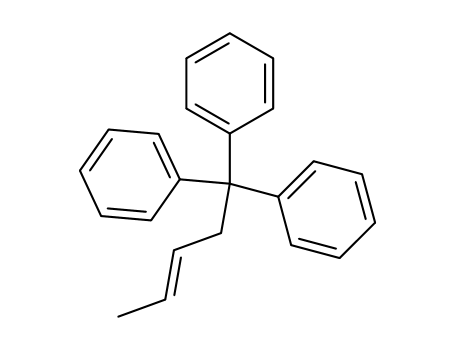
(E)-pent-3-ene-1,1,1-triyltribenzene
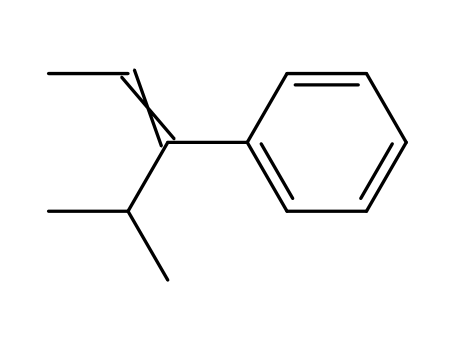
4-methyl-3-phenyl-pent-2ξ-ene
(2-hydroxyimino-1-methyl-2-phenyl-ethyl)-triphenyl-phosphonium; bromide
CAS:1779-49-3
CAS:1779-51-7
CAS:2128-93-0
CAS:56-93-9