Your Location:Home >Products >Organic Chemistry >51554-95-1


Product Details
|
Chemical Properties |
Clear pale yellow liquid |
|
Uses |
4-Pentylbromobenzene is a derivative of Valerophenone (V091450), which is an aromatic ketone that is often used as a tool in the study of various photochemical processes. Valerophenone is also an inhibitor of the enzyme carbonyl reductase. |
InChI:InChI=1/C11H15Br/c1-2-3-4-5-10-6-8-11(12)9-7-10/h6-9H,2-5H2,1H3
Under mild conditions (room temperature,...
The invention belongs to the technical f...
B(C6F5)3-catalyzed hydrodesulfurization ...
Two series of bis[1,2-bis(4-n-alkylpheny...
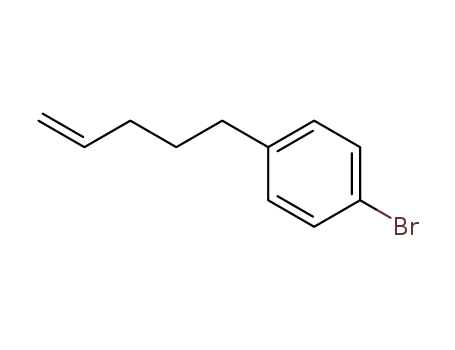
1-bromo-4-(pent-4-en-1-yl)benzene


4-pentylbromobenzene
| Conditions | Yield |
|---|---|
|
With
(η5-C5Me5)Rh(ppy)H; hydrogen;
In
methanol;
at 20 ℃;
for 24h;
under 4137.29 Torr;
|
95% |
C17H18BrClS


4-pentylbromobenzene
| Conditions | Yield |
|---|---|
|
With
triethylsilane; tris(pentafluorophenyl)borate;
In
chloroform-d1;
at 20 ℃;
for 72h;
chemoselective reaction;
|
83% |
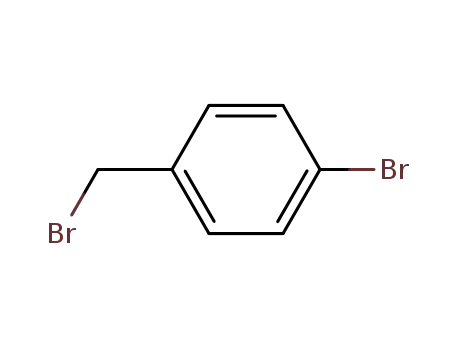
1-bromomethyl-4-bromobenzene
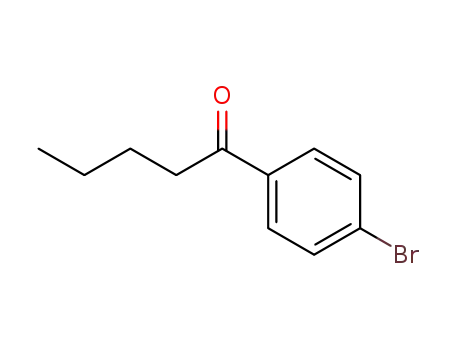
4'-bromovalerophenone

4-(1-trans-pentenyl)-bromobenzene
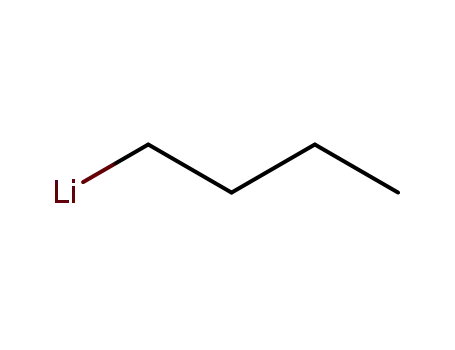
n-butyllithium
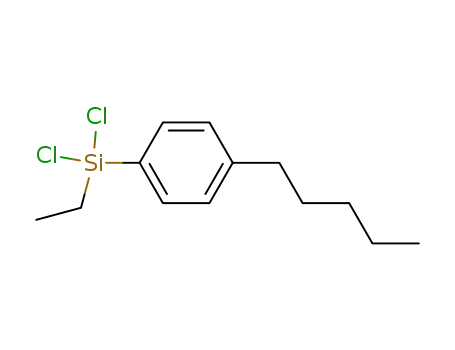
(dichloro)(ethyl)<4-(pentyl)phenyl>silane
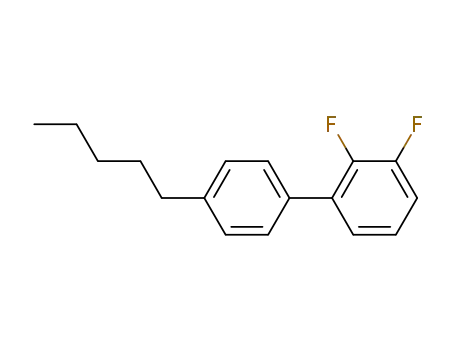
2,3-difluoro-4'-pentylbiphenyl
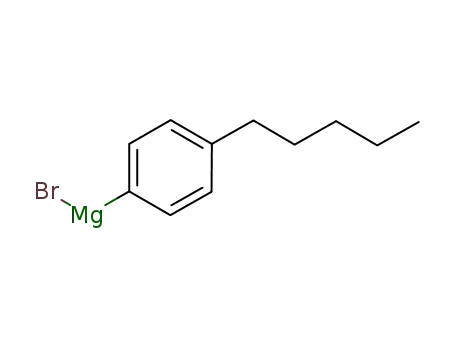
p-pentylphenylmagnesium bromide
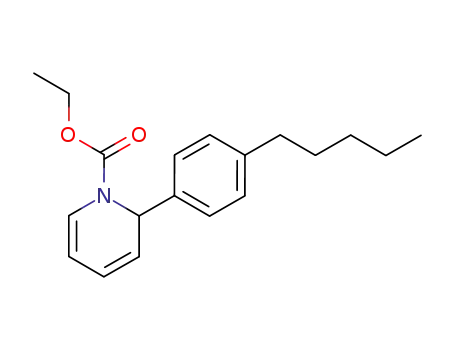
2-(4-pentyl-phenyl)-2H-pyridine-1-carboxylic acid ethyl ester
CAS:156573-09-0
CAS:1585-07-5
CAS:41492-05-1
CAS:1073-06-9