Your Location:Home >Products >Organic Chemistry >41492-05-1
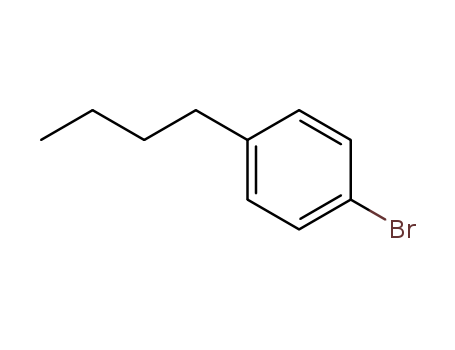

Product Details
|
Chemical Properties |
clear colorless to yellow liquid |
|
Uses |
1-Bromo-4-butylbenzene was used in the preparation of:4-(4-butylphenyl)-2-methylbut-3-yn-2-olteraaza- and hexaazacyclophane4-butyltriphenylamine, monomer required for the preparation of poly(4-butyltriphenylamine) |
|
General Description |
Phosphite or phosphine oxide ligand catalyzed Suzuki-Miyaura reaction of 2-pyridyl boron derivatives with 1-bromo-4-butylbenzene has been reported. |
|
Flammability and Explosibility |
Notclassified |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C10H13Br/c1-2-3-4-9-5-7-10(11)8-6-9/h5-8H,2-4H2,1H3
The invention belongs to the technical f...
An operationally simple and environmenta...
Abnormal reactivity has been observed in...
The palladium-catalyzed conversion of ar...
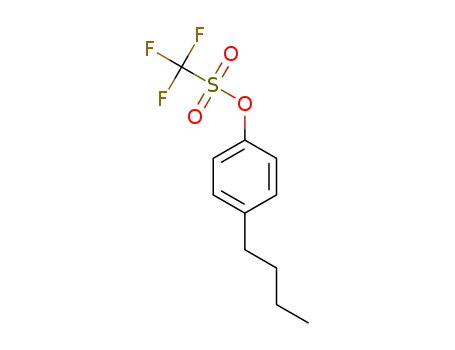
4-n-butylphenyl trifluoromethanesulfonate

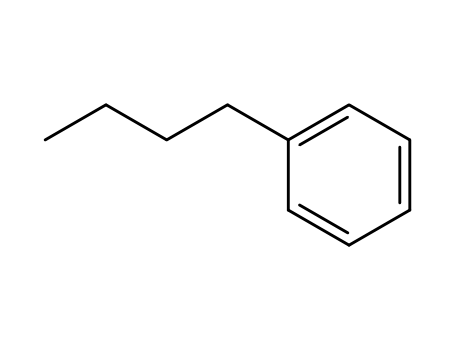
1-butylbenzene

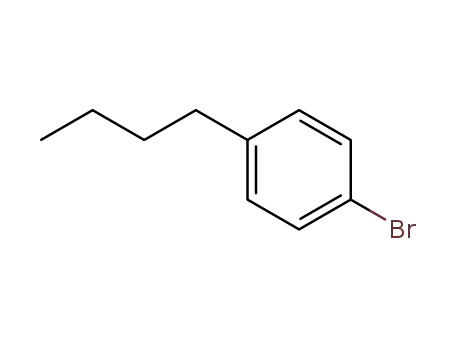
p-bromobutylbenzene
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); triisopropylaluminum diethyl etherate; t-BuBrettPhos; potassium bromide;
In
toluene;
at 100 ℃;
Inert atmosphere;
|
25 %Spectr. |
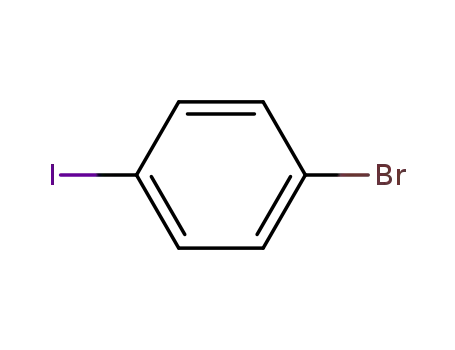
1,4-bromoiodobenzene


tri-n-butylindium


p-bromobutylbenzene
| Conditions | Yield |
|---|---|
|
With
bis-triphenylphosphine-palladium(II) chloride;
In
tetrahydrofuran;
for 4h;
Heating;
|
80% |
|
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran;
Heating;
|
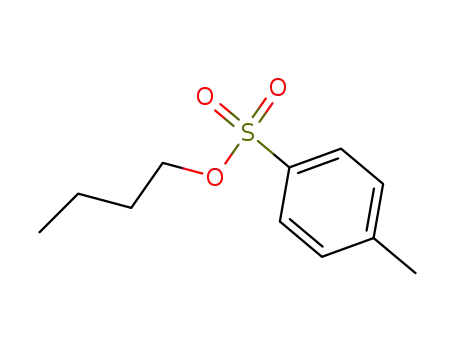
butyl para-toluenesulfonate
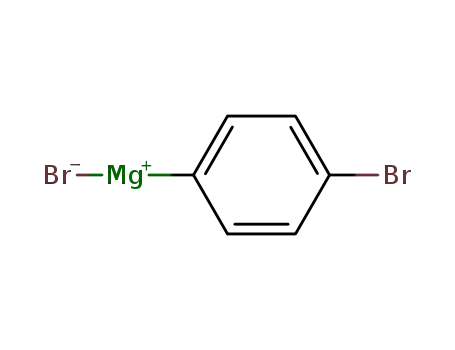
(4-bromophenyl)magnesium bromide
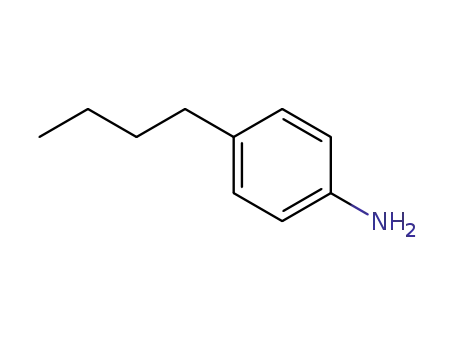
4-Butylaniline

1-butylbenzene
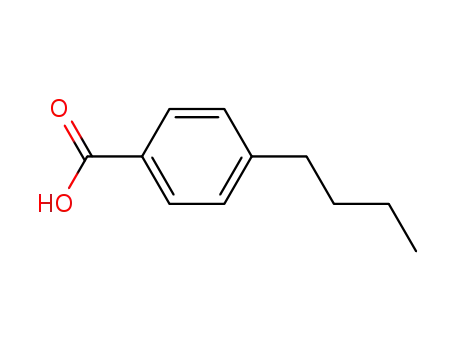
4-butyl benzoic acid
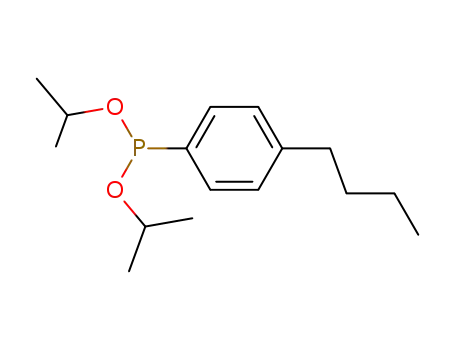
C16H27O2P

Tris-(4-butyl-phenyl)-phosphane
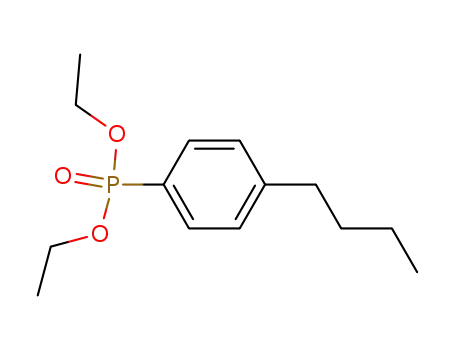
(4-Butyl-phenyl)-phosphonic acid diethyl ester
CAS:156573-09-0
CAS:1585-07-5
CAS:588-93-2
CAS:51554-95-1