Your Location:Home >Products >Chemical Reagents >134-85-0

Product Details
|
Chemical Properties |
White to off-white powder |
|
Uses |
UV curing type coating, printing ink, medical and pesticide intermediate |
|
Definition |
ChEBI: 4-chlorobenzophenone is a member of benzophenones. It is a known transformation product of 1-[(4-Chlorophenyl)phenylmethyl]piperazine, Cetirizine, Cetirizine N-oxide, Chlorcyclizine, and Meclozine. |
|
Synthesis Reference(s) |
Tetrahedron Letters, 40, p. 3109, 1999 DOI: 10.1016/S0040-4039(99)00476-1 |
|
Purification Methods |
Recrystallise it from EtOH. [Wagner et al. J Am Chem Soc 108 7727 1986, Beilstein 7 H 419, 7 I 227, 7 II 359, 7 III 2072, 7 IV 1375.] |
InChI:InChI=1/C13H9ClO/c14-12-8-6-11(7-9-12)13(15)10-4-2-1-3-5-10/h1-9H
Polydopamine/MIL-53(Fe) (PDA/MIL-53(Fe))...
In this study, new RuO2@ZrO2 core-shell ...
A range of metal bis{(trifluoromethyl)su...
Chromium containing medium pore molecula...
-
-
An environmentally friendly aerobic oxid...
Although cross-coupling reactions of ami...
A Rh(I)-catalyzed ketone Suzuki-Miyaura ...
A novel metal-organic framework (MOF) wi...
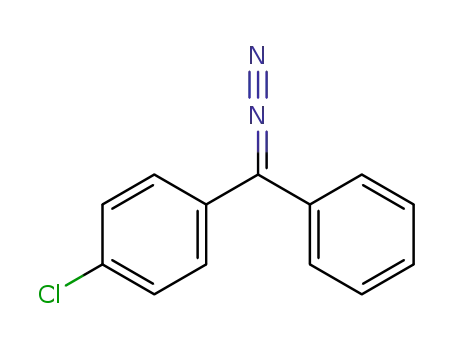
1-chloro-4-(diazo(phenyl)methyl)benzene

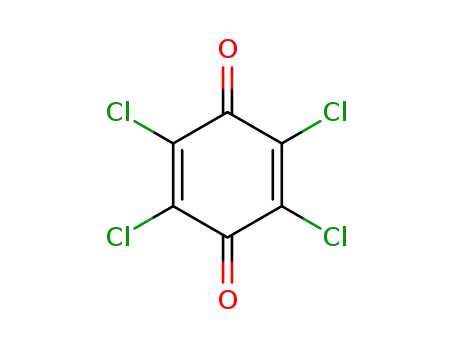
chloranil

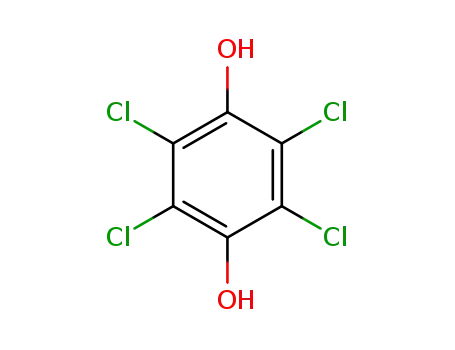
2,3,5,6-tetrachlorobenzene-1,4-diol

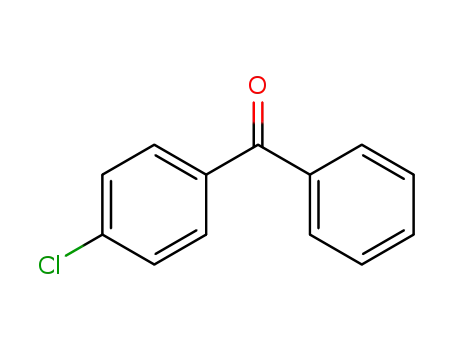
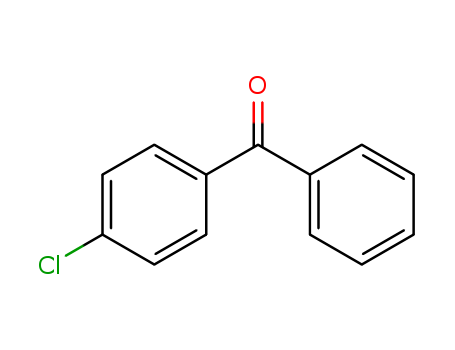
4-chlorobenzophenone
| Conditions | Yield |
|---|---|
|
With
water;
In
tetrahydrofuran;
at 30 ℃;
Rate constant;
Kinetics;
Mechanism;
various temperatures, Δ H(excit.), Δ S(excit.), energy data;
|

benzonitrile

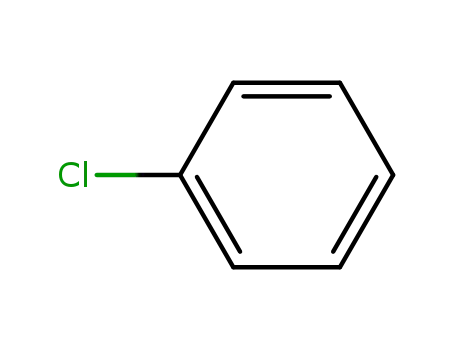
chlorobenzene

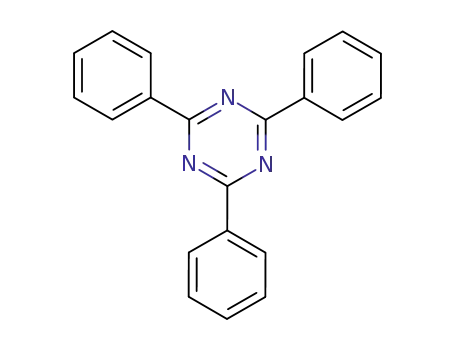
2,4,6-triphenyl-1,3,5-triazine


4-chlorobenzophenone
| Conditions | Yield |
|---|---|
|
trifluorormethanesulfonic acid;
|
21% |
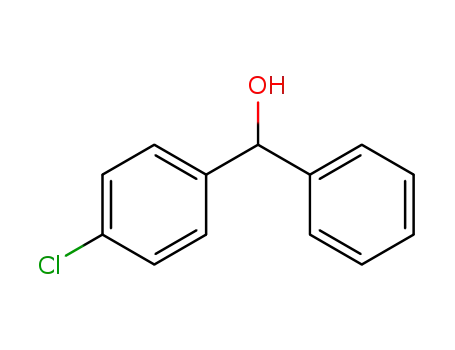
(4-chlorophenyl)phenylmethanol
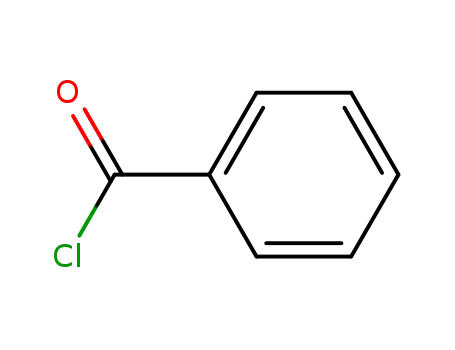
benzoyl chloride
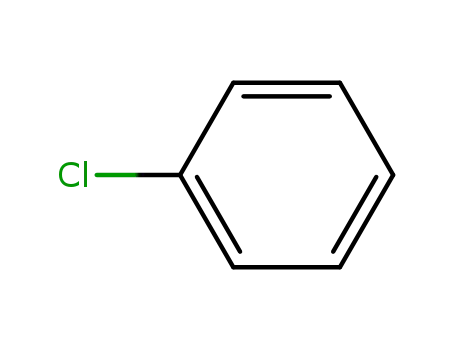
chlorobenzene
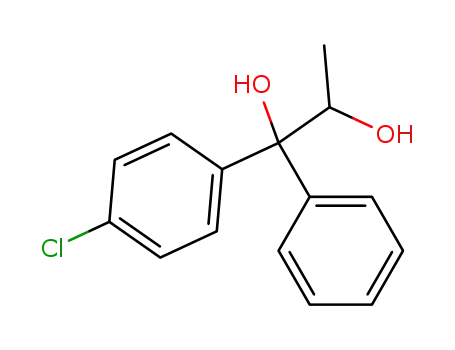
1-(4-chloro-phenyl)-1-phenyl-propane-1,2-diol
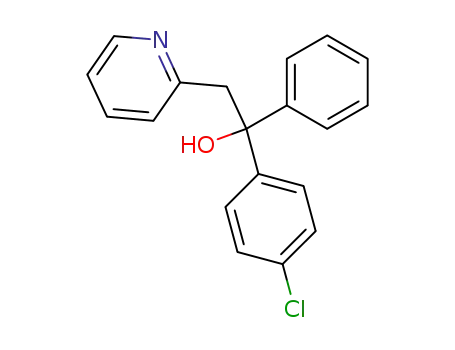
1-(4-chloro-phenyl)-1-phenyl-2-[2]pyridyl-ethanol
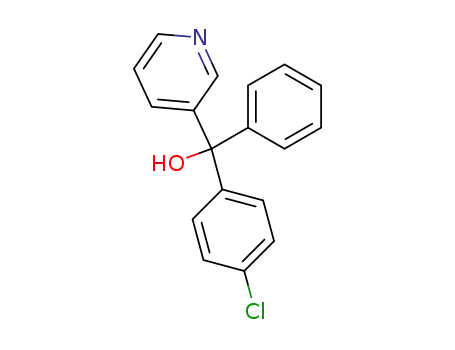
(4-chloro-phenyl)-phenyl-[3]pyridyl-methanol
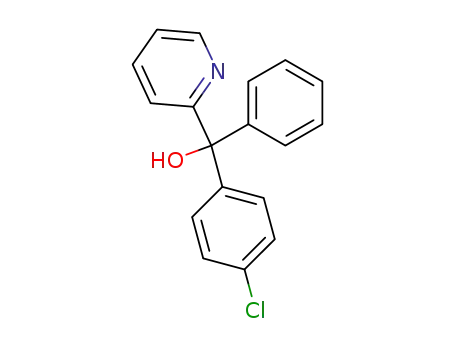
(4-chloro-phenyl)-phenyl-[2]pyridyl-methanol
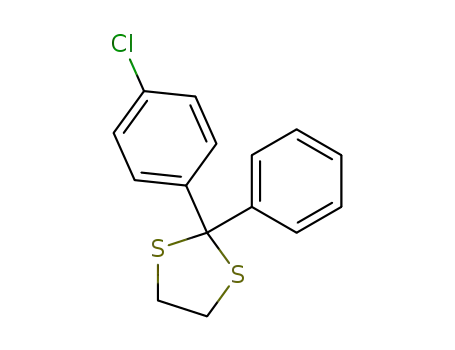
2-(4-chlorophenyl)-2-phenyl-1,3-dithiolane
CAS:4009-98-7
CAS:625-36-5
CAS:2420-87-3
CAS:2128-93-0