Your Location:Home >Products >Chemical Reagents >625-36-5
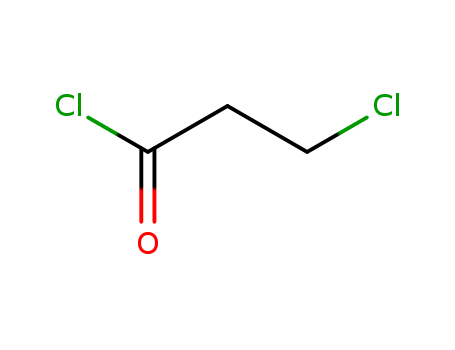

Product Details
|
Description |
3-Chloropropionyl chloride is an important bifunctional reagent. It is capable of acylation and possesses a 2-chloro-ethyl fragment (CH2CH2Cl), which can be subjected to nucleophilic substitution and serves as a masked vinyl group. It can be used as a starting material in many reactions to construct a variety of (hetero)cyclic compounds. |
|
Chemical Properties |
Clear colorless to dark brown liquid. Soluble in ethanol, ether and chloroform. Slightly soluble in water. |
|
Uses |
3-Chloropropionyl chloride is a reagent used in the synthesis of Beclamide (B119400), which is a chlorinated benzylpropanamide used as an anticonvulsant drug. It is used in the treatment of tonic-clonic seizyres and has sedative properties. |
|
Preparation |
3-Chloropropionyl chloride (1) is commercially available and can be prepared from β-propiolactone (2) and thionyl chloride.1 Other standard methods available for the preparation of acyl chlorides can also be applied: the reaction of acrylic acid (3) or 3-chloropropionic acid (4) with thionyl chloride, phosphoryl chloride, phosgene, or phosphorus trichloride. |
|
Reactions |
(1) The Friedel–Crafts acylation of tert-butylbenzene (5) with 3-chloropropionyl chloride (1) followed by cyclization provid- ed indanone 6, which was further transformed into urea derivative 7, a potent TRPV1 antagonist. (2) A novel high-yielding one-pot microwave-assisted synthesis of condensed 5-substituted pyranoisoquinoline-1,6-diones 9 from 2-substituted isoquinoline-1,3-diones 8 and 3-chloropropionyl chloride (1) was reported. (3) Acylation of 2-aminophenol (12) with 3-chloropropionyl chloride (1), followed by cyclization in the presence of polyphosphoric acid (PPA), gave benzoxazole 13, which was further reacted with 4-chlorophenyl-1-piperazine to yield the target benzo[d]oxazole analogue 14, a selective dopamine D4 receptor ligand. |
|
Flammability and Explosibility |
Nonflammable |
|
Synthesis |
3-Chloropropionyl chloride is produced by reaction of acrylic acid with hydrogen chloride and phosgene in the presence of, for example,dimethylformamide as catalyst, or by reaction of propiolactone with thionyl chloride. |
InChI:InChI=1/C3H4Cl2O/c4-2-1-3(5)6/h1-2H2
Dicobaltohexacarbonyl 4-methyl-pent-3-en...
The invention discloses a method for pro...
The invention relates to the technical f...
The invention provides a preparation met...
The invention relates to a preparation m...
acrylic acid

2-chloropropionyl chloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; thionyl chloride; 10H-phenothiazine;
In
water;
at 40 - 75 ℃;
for 13h;
Temperature;
|
92.5% |
|
With
phosgene;
N,N-dimethyl-formamide;
at 70 ℃;
for 10h;
|
80% |
|
With
N,N-dimethyl-formamide;
|
|
|
With
phosgene;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 40h;
|
|
|
With
phosgene;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 40h;
|
200 g |
|
With
thionyl chloride;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 0.416667h;
Concentration;
Reagent/catalyst;
|
β-Propiolactone


2-chloropropionyl chloride
| Conditions | Yield |
|---|---|
|
With
bis(trichloromethyl) carbonate; N,N-dimethylpiperidine-4-carboxamide hydrochloride;
at 25 ℃;
for 10h;
Reagent/catalyst;
Large scale;
|
98% |
|
With
phosphorus pentachloride;
In
benzene;
at 20 ℃;
for 10h;
|
87% |
|
With
tetrachloromethane; sulfuryl dichloride;
|
|
|
With
tetrachloromethane; phosphorus pentachloride;
|
|
|
With
thionyl chloride;
|
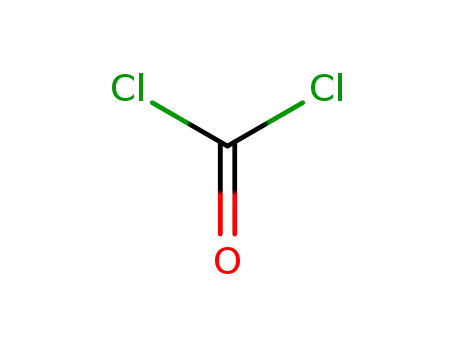
phosgene
ethene
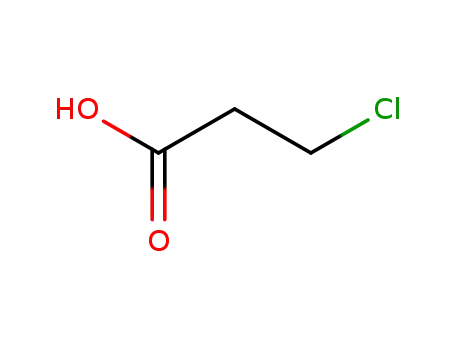
chloropropionic acid

β-Propiolactone
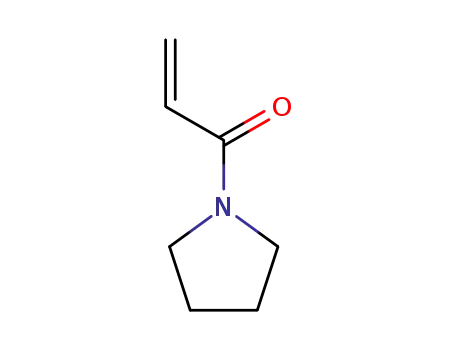
N-acryloylpyrrolidine
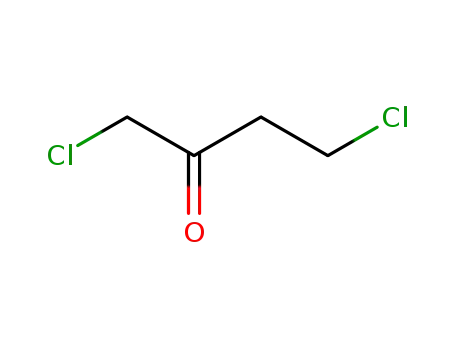
1,4-dichloro-2-butanone
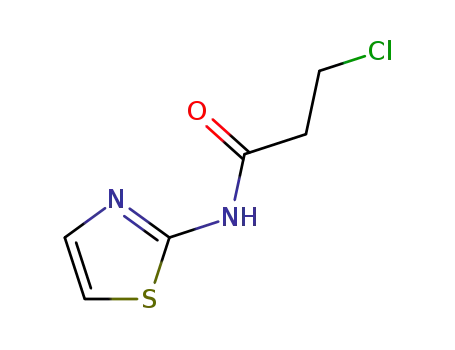
3-chloro-N-(1,3-thiazol-2-yl)propanamide
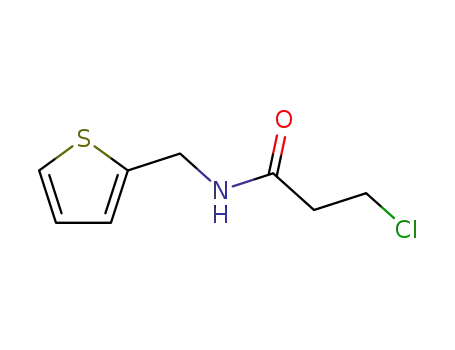
3-chloro-propionic acid-([2]thienylmethyl-amide)
CAS:4009-98-7
CAS:134-85-0
CAS:60811-21-4
CAS:86579-53-5