Your Location:Home >Products >Organic Chemistry >107-05-1
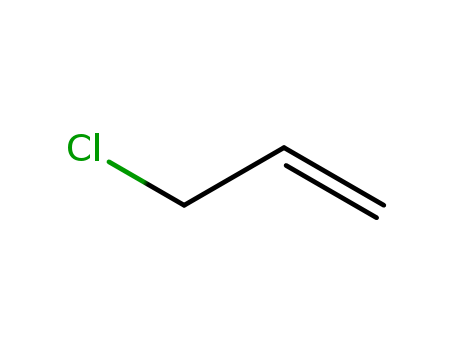
Product Details
|
Chemical Properties |
colourless, light yellow or amber liquid with an unpleasant smell |
|
Physical properties |
Colorless to light brown to reddish-brown liquid with a pungent, unpleasant, garlic-like odor. An experimentally determined odor threshold concentration of 470 ppbv was reported by Leonardos et al. (1969). |
|
Uses |
Allyl chloride (3-chloropropene; 1-chloro-2-propene) is a chemical intermediate used in the synthesis of allyl compounds found in varnish, resins, polymers, pesticides, and pharmaceuticals (O’Neil, 2001). |
|
Production Methods |
Allyl chloride can be synthesized by reaction of allyl alcohol with HCl or by treatment of allyl formate with HCl in the presence of a catalyst (ZnCl2). |
|
General Description |
A clear colorless liquid with an unpleasant pungent odor. Flash point -20°F. Boiling point 113°F. Less dense than water (7.8 lb / gal) and insoluble in water. Hence floats on water. Vapor irritates skin, eyes and mucous membranes. Vapors are heavier than air. Long exposure to low concentrations or short exposure to high concentrations may have adverse health effects from inhalation or skin absorption. |
|
Air & Water Reactions |
Highly flammable. Insoluble in water. |
|
Reactivity Profile |
Allyl chloride presents a serious fire and explosion hazard when exposed to heat, flame or oxidizing agents. Polymerizes violently and exothermically with Lewis acids (aluminum chloride, boron trifluoride, sulfuric acid) or metals (aluminum, magnesium, zinc, or galvanized metal) [MCA SD-99, 1973]. Incompatible with acids (nitric acid, chlorosulfonic acid, oleum), with strong bases (sodium hydroxide, potassium hydroxide), with ethyleneimine and ethylenediamine [Lewis, 3rd ed., 1993, p. 36]. Attempts to alkylate benzene or toluene using Allyl chloride in the presence of ethylaluminum chlorides have led to explosions. |
|
Hazard |
Skin and eye irritant. Upper respiratory tract irritant, liver and kidney damage. Question- able carcinogen. |
|
Health Hazard |
Causes marked irritation of skin and may burn. Burns the eyes; effect may be delayed. |
|
Fire Hazard |
Special Hazards of Combustion Products: Releases irritating hydrogen chloride gas on combustion |
|
Flammability and Explosibility |
Highlyflammable |
|
Safety Profile |
Suspected carcinogen with experimental tumorigenic data. Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion, inhalation, and skin contact. Experimental teratogenic and reproductive effects. A skin and eye irritant. Human mutation data reported. Chronic exposure may cause liver and lildney damage. The vapors of allyl chloride are quite irritating to the eyes, nose, and throat. Contact of the liquid with the skin may lead, in addition to local vasoconstriction and numbness, to rapid absorption and distribution through the body. If remedial measures are not taken promptly, such contact may result in burns and internal injuries. Inhalation may cause headache, dizziness, and in htgh concentration, loss of consciousness; however, even in low concentration, its odor in most cases is irritating enough to give warning of its presence. Concentration of the vapors high enough to cause serious effects, includlng damage to the lungs, especially on repeated exposure, may not be intolerable. Consequently, the warning characteristics should never be disregarded. In general, precautions should be taken AT ALL TIMES to avoid splllage and accumulation of noticeable concentration of the vapors in the atmosphere. Acute exposure in experimental animals has resulted in marked inflammation of lungs, irritation of skin, and swelling of the hdneys. Chronically exposed animals have shown degenerative changes in the liver and kidneys. Reported human exposures have been principally cases of irritation of the eyes, skin, and respiratory tract, sometimes accompanied by aches and pains in the bones. Liver and hdney injury is possible. Dangerous fire and explosion hazard when exposed to heat, flame, or oxidlzers. Vigorous or explosive reaction above -7O℃ with alkyl aluminum chlorides (e.g., trichlorotriethyl dialuminum, ethyl aluminum dichloride, or diethyl aluminum chloride) + aromatic hydrocarbons (e.g., benzene or toluene). Violently exothermic polymerization reaction with Lewis acids (e.g., aluminum chloride, boron trifluoride, or sulfuric acid) and metals (e.g., aluminum, magnesium, zinc, or galvanized metals). Incompatible with HNO3, ethylene imine, ethylenedlamine, chlorosulfonic acid, oleum, NaOH. To fight fire, use CO2, alcohol foam, dry chemical. See also CHLORINATED HYDROCARBONS; ALIPHATIC; ALLYL COMPOUNDS; and CHLORIDES. Storage and Handling: Keep cool, away from heat sources. Maintain good vendation. Work in a fume hood or with closed system if possible; otherwise, use adequate vendation so that the odor of allyl chloride does not persist. If it should be necessary to enter an area in which the odor of allyl chloride is at all noticeable, use a gas mask equipped with an “organic vapor” canister. Do not dlsregard the warning odor or eye irritation of allyl chloride |
|
Potential Exposure |
Allyl chloride is used as a chemical intermediate and in making allyl compounds, epichlorohydrin, and glycerol. |
|
Carcinogenicity |
The IARC found that it could not classify AC as a human carcinogen on the basis of available data. In contrast, EPA considers AC to be a possible human carcinogen and has ranked it in EPA’s Group C. This classification was based on a low incidence of forestomach tumors in female mice and positive results in a variety of genetic toxicity tests. However, the forestomach tumor data were not used for quantitative cancer risk assessment. AC is a strong alkylating agent and is structurally similar to other forestomach carcinogens, such as propylene oxide and epichlorohydrin, which cause tumors at the site of exposure. Olsen reported on a cohort of 1064 men employed at a Texas plant in epoxy resin, glycerin, andAC/epichlorohydrin production between 1957 and 1986 and followed up through 1989. There were 66 total deaths [standardized mortality ratio (SMR)=0.8; 95% CI 0.6–1.0] and 10 cancers (SMR=0.5; CI 0.2–0.9).However, the authors noted that the cohort was limited due to sample size, duration of follow-up, small numbers of deaths both expected and found, and the limited exposure potential. |
|
Environmental fate |
Biological. Bridié et al. (1979) reported BOD and COD values of 0.23 and 0.86 g/g using filtered effluent from a biological sanitary waste treatment plant. These values were determined using a standard dilution method at 20 °C and stirred for a period of 5 d. When a sewage seed was used in a separate screening test, a BOD value of 0.42 g/g was obtained. The ThOD for allyl chloride is 1.67 g/g. Photolytic. Anticipated products from the reaction of allyl chloride with ozone or OH radicals in the atmosphere are formaldehyde, formic acid, chloroacetaldehyde, chloroacetic acid, and chlorinated hydroxy carbonyls (Cupitt, 1980). Chemical/Physical. Hydrolysis under alkaline conditions will yield allyl alcohol (Hawley, 1981). The estimated hydrolysis half-life in water at 25 °C and pH 7 is 2.0 yr (Mabey and Mill, 1978). |
|
Shipping |
UN1100 Allyl chloride, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous materials |
|
Purification Methods |
Likely impurities include 2-chloropropene, propyl chloride, iso-propyl chloride, 3,3-dichloropropane, 1,2-dichloropropane and 1,3-dichloropropane. Purify it by washing with conc HCl, then with Na2CO3 solution, dry it with CaCl2, and distil it through an efficient column [Oae & Vanderwerf J Am Chem Soc 75 2724 1953]. [Beilstein 1 IV 738.] LACHRYMATORY, TOXIC. |
|
Incompatibilities |
Contact with water forms hydrochloric acid. Keep away from strong oxidizers, acids, aluminum, amines, peroxides, chlorides of iron and aluminum; magnesium, zinc. |
|
Waste Disposal |
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration at a temperature of 982 C for 2 seconds minimum. |
|
EXPOSURE ROUTES |
inhalation, skin absorption, ingestion, skin and/or eye contact |
|
FIRST AID |
(See procedures) Eye:Irrigate immediately Skin:Soap wash immediately Breathing:Respiratory support Swallow:Medical attention immediately |
Radical-promoted tributyltin hydride red...
The thermal decomposition reaction of 1,...
Several examples of synthetically unique...
The rate constant for the reaction Cl + ...
The kinetics of the reactions of three u...
Dehydrochlorination of 1,2-dichloropropa...
(Chemical Equation Presented) The decomp...
Inclusion compounds of di-μ-chloro-bis(η...
-
Silphos [PCl3-n(SiO2)n] as a heterogeneo...
The ready decomposition of the vinyl-di-...
The epoxidation of olefin as a strategy ...
The invention belongs to the technical f...
Two strategies were reported to prevent ...
The invention discloses a chlorinating p...
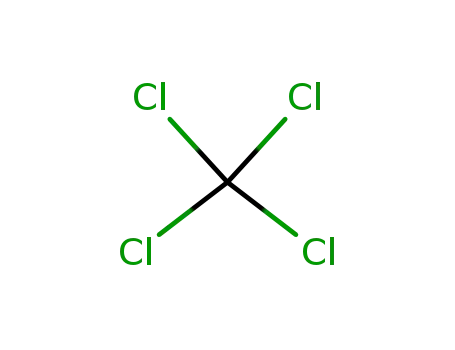
tetrachloromethane

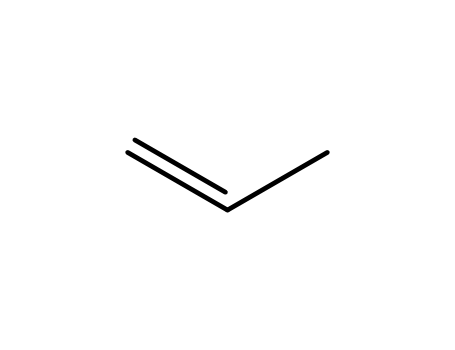
propene

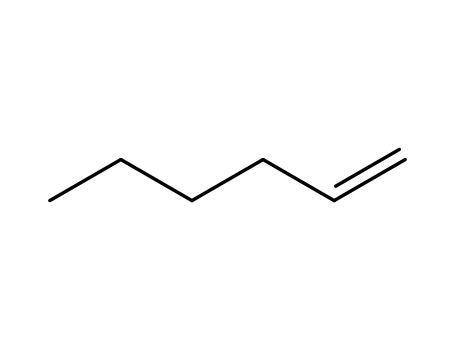
1-hexene

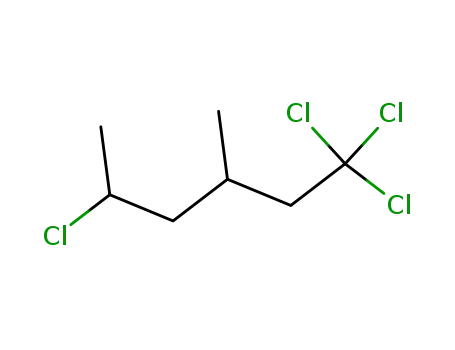
1,1,1,5-tetrachloro-3-methylhexane

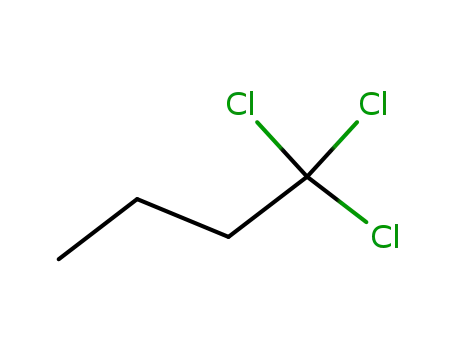
1,1,1-trichloro-butane

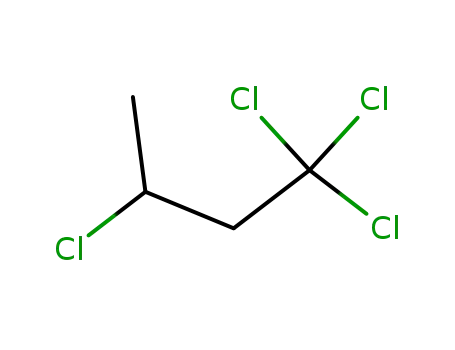
1,1,1,3-tetrachlorobutane

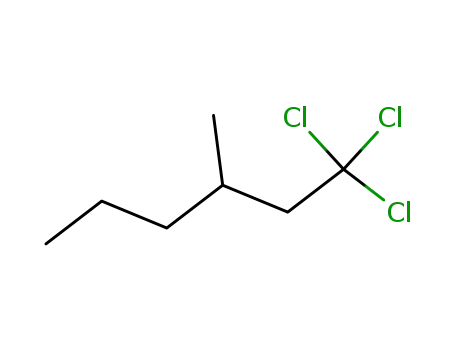
1,1,1-trichloro-3-methyl-hexane

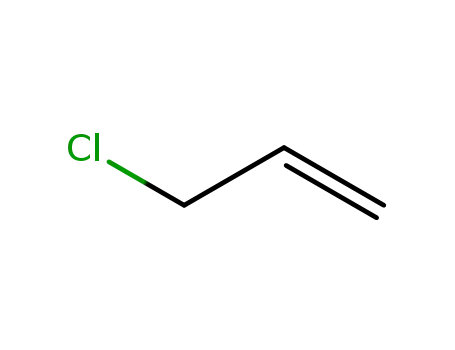
3-chloroprop-1-ene
| Conditions | Yield |
|---|---|
|
With tungsten hexacarbonyl; at 140 ℃; for 1h; Product distribution; Mechanism; chain termination in the telomerization of title compound;
|
1 % Chromat. 1 % Chromat. |
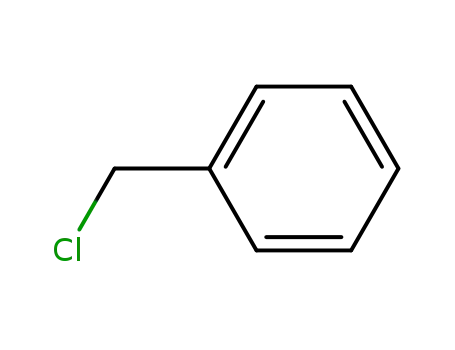
benzyl chloride

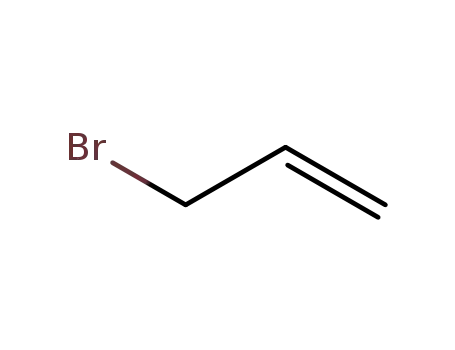
allyl bromide

benzyl bromide

3-chloroprop-1-ene
| Conditions | Yield |
|---|---|
|
With tetrabutylammomium bromide; In nitromethane-d3; at 25 ℃; Equilibrium constant; Thermodynamic data; ΔG;
|
pyridine
benzenesulfonyl chloride
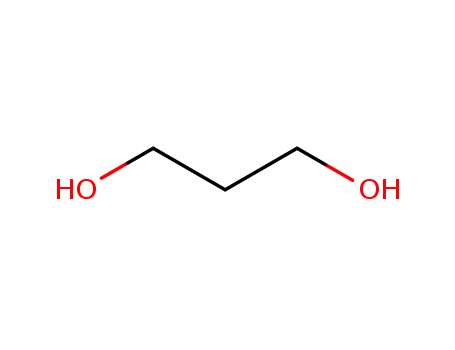
trimethyleneglycol

propene
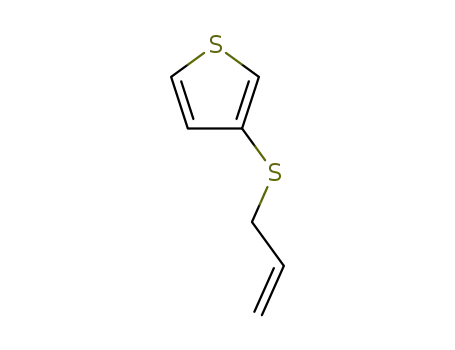
allyl-3-thienyl sulfide
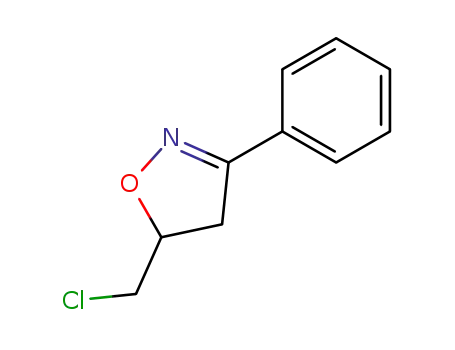
5-(chloromethyl)-3-phenyl-4,5-dihydro-1,2-oxazole
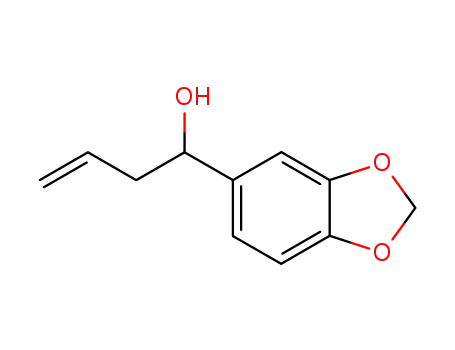
1-benzo[1,3]dioxol-5-yl-but-3-en-1-ol
7-allyl-1,3-dimethylxanthine
CAS:4009-98-7
CAS:134-85-0
CAS:56-37-1
CAS:60811-21-4