Your Location:Home >Products >Surfactant >2495-39-8
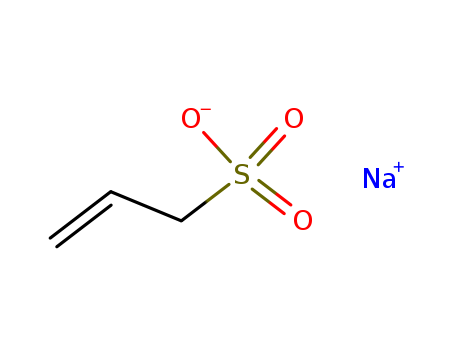

Product Details
|
Chemical Properties |
White to Almost white powder to crystal. |
|
Uses |
Sodium allyl sulfonate is used as a basic brightener in nickel electroplating baths. It is also used as pharmaceutical intermediates. |
|
Flammability and Explosibility |
Nonflammable |
InChI:InChI=1/C3H6O3S/c1-2-3-7(4,5)6/h2H,1,3H2,(H,4,5,6)/p-1
Micellar catalysis with various polyoxye...
Ring closing metathesis (RCM) using Grub...
The invention discloses a synthesis meth...
Carbanions of sulfonyl halides and activ...
Provided herein are dUTPase inhibitors, ...
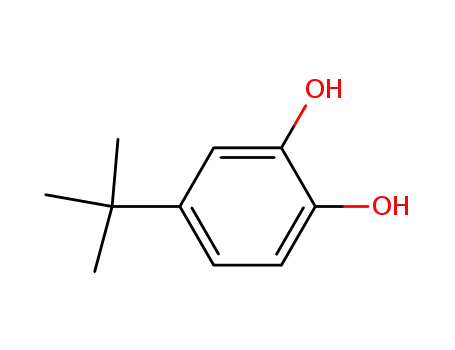
4-tert-Butylcatechol

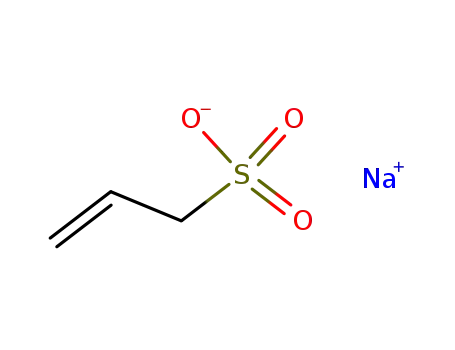
sodium prop-2-ene-1-sulfonate
| Conditions | Yield |
|---|---|
|
With sodium metabisulfite; N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide; In water; at 40 - 50 ℃; under 2250.23 Torr; Temperature; Reagent/catalyst; Pressure;
|
98.93% |
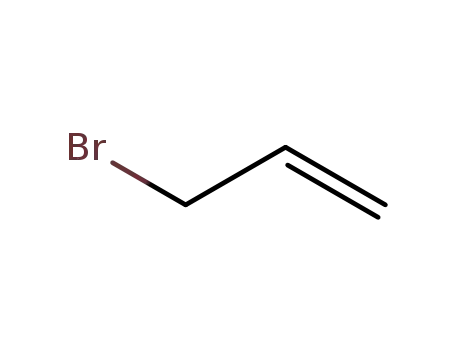
allyl bromide


sodium prop-2-ene-1-sulfonate
| Conditions | Yield |
|---|---|
|
With sodium sulfite;
|
78% |
|
With sodium sulfite; In ethanol; water; for 3h; Heating / reflux;
|
76% |
|
With sodium sulfite; In ethanol; water; for 2.5h; Heating;
|
75% |
|
With sodium sulfite; In water; for 24h; Reflux;
|
|
|
With sodium sulfite; In water; at 100 ℃;
|
|
|
With sodium sulfite; In water; at 100 ℃; for 16h;
|
|
|
With sodium sulfite; In water; for 8h; Reflux;
|
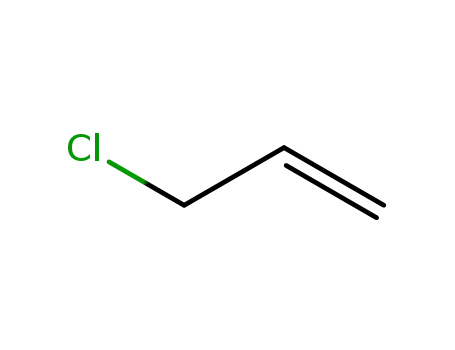
3-chloroprop-1-ene
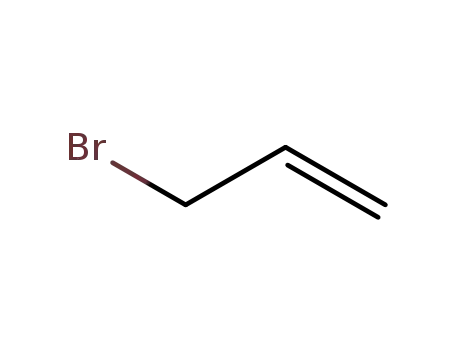
allyl bromide
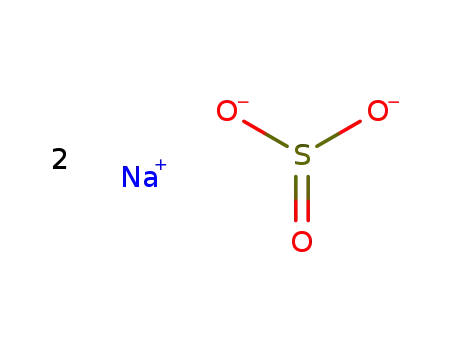
sodium sulfite

4-tert-Butylcatechol
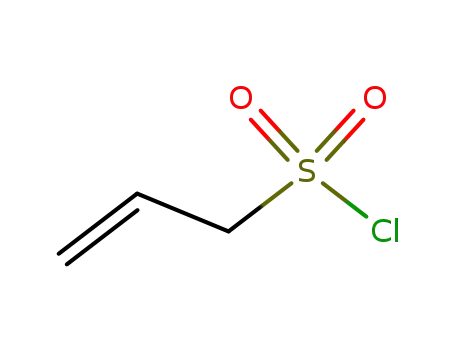
allylsulfonyl chloride
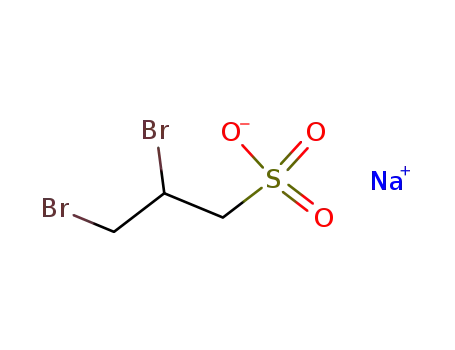
sodium 2,3-dibromopropane-1-sulfonate
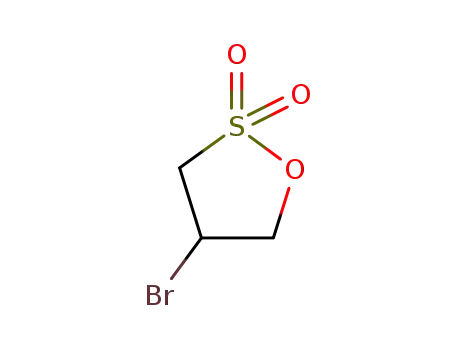
2-bromopropane-1,3-sultone
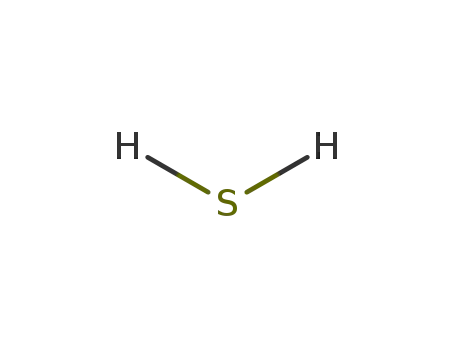
hydrogen sulfide
CAS:1561-92-8
CAS:22047-25-2
CAS:60811-18-9
CAS:126-33-0