Your Location:Home >Products >Organic Chemistry >1765-93-1
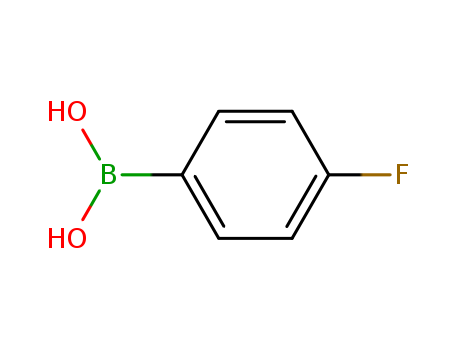

Product Details
Amyloidogenic peptides are considered ce...
Fully automated synthetic chemistry woul...
The invention belongs to the technical f...
Asymmetric 1,4-addition reactions with β...
In this study, we developed a simple tra...
C16H16BFO2

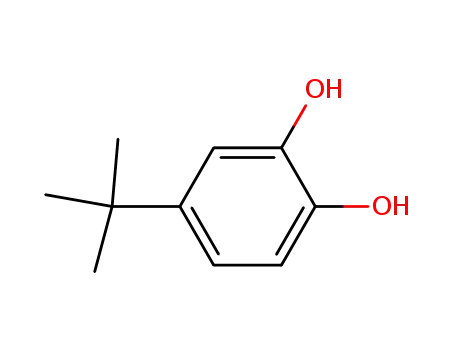
4-tert-Butylcatechol

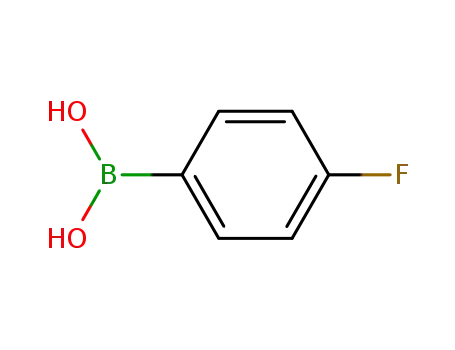
4-fluoroboronic acid
| Conditions | Yield |
|---|---|
|
In
chloroform-d1;
at 25 ℃;
Equilibrium constant;
Sealed tube;
|
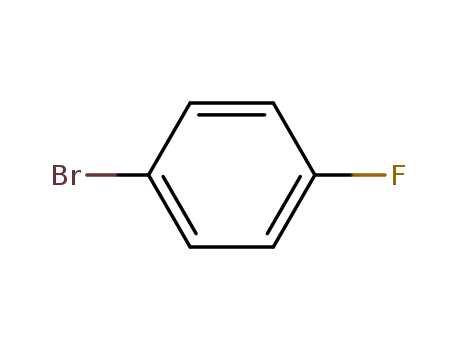
1-Bromo-4-fluorobenzene


4-fluoroboronic acid
| Conditions | Yield |
|---|---|
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -60 ℃;
|
77% |
|
1-Bromo-4-fluorobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 ℃;
|
62% |
|
1-Bromo-4-fluorobenzene;
With
tert.-butyl lithium;
In
tetrahydrofuran;
at -78 ℃;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
Further stages.;
|
59% |
|
1-Bromo-4-fluorobenzene;
With
tris(dibenzylideneacetone)dipalladium(0) chloroform complex; diisopropopylaminoborane; triethylamine; triphenylphosphine;
In
tetrahydrofuran;
at 65 ℃;
for 12h;
Inert atmosphere;
With
methanol;
In
tetrahydrofuran;
at 0 ℃;
Further stages;
Inert atmosphere;
|
40% |
|
1-Bromo-4-fluorobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.25h;
Inert atmosphere;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 30h;
Inert atmosphere;
With
hydrogenchloride;
In
tetrahydrofuran; hexane; water;
Inert atmosphere;
|
37% |
|
1-Bromo-4-fluorobenzene;
With
magnesium;
In
diethyl ether;
With
Trimethyl borate;
In
diethyl ether;
at 3 ℃;
for 2h;
With
hydrogenchloride;
In
diethyl ether;
|
|
|
1-Bromo-4-fluorobenzene;
With
magnesium;
In
tetrahydrofuran;
Heating;
With
Trimethyl borate;
In
tetrahydrofuran;
at 20 ℃;
|
|
|
1-Bromo-4-fluorobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane; toluene;
at -70 ℃;
for 1.5h;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -70 - -20 ℃;
Further stages.;
|
91 % Chromat. |
|
With
Trimethyl borate;
|
|
|
1-Bromo-4-fluorobenzene;
With
magnesium;
In
tetrahydrofuran;
With
Trimethyl borate;
With
hydrogenchloride;
Further stages.;
|
|
|
With
tetrahydroxydiboron; (chloro(2-dicyclohexylphosphino-2′,4′,6′-triisopropyl-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II)); potassium acetate; XPhos;
In
ethanol;
at 80 ℃;
for 4h;
Inert atmosphere;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran / 1 h / -78 °C / Inert atmosphere; Schlenk technique
1.2: -78 - 20 °C / Inert atmosphere; Schlenk technique
2.1: hydrogenchloride / water / 20 °C / Inert atmosphere; Schlenk technique
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran; water;
|
|
|
With
tetrahydroxydiboron; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N-ethyl-N,N-diisopropylamine; triphenylphosphine;
In
ethanol;
at 20 ℃;
for 12h;
Inert atmosphere;
Sealed tube;
|
|
|
1-Bromo-4-fluorobenzene;
With
magnesium;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
In
toluene;
for 1h;
Reflux;
Dean-Stark;
|
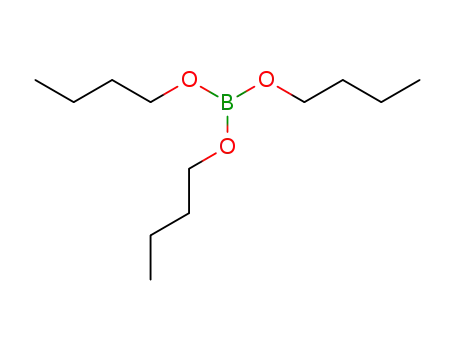
boric acid tributyl ester
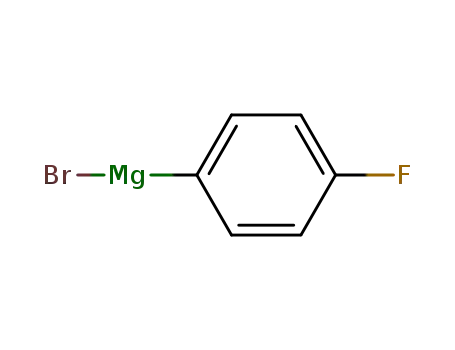
4-flourophenylmagnesium bromide
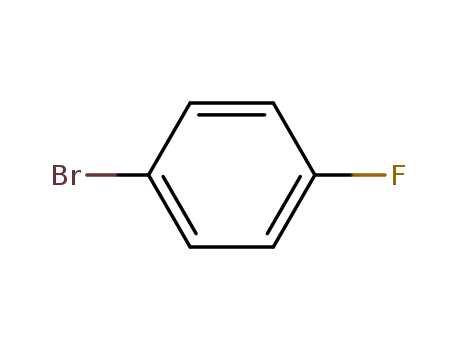
1-Bromo-4-fluorobenzene
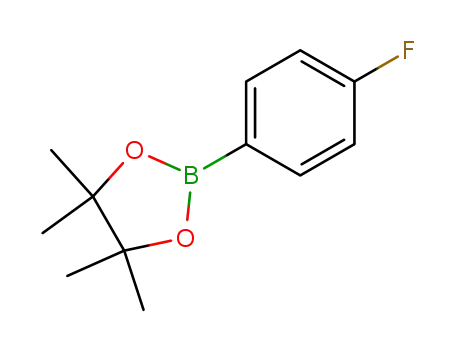
2-(4-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
tris(4-fluorophenyl)boroxine
3-(4-fluorophenyl)-4H-chromen-4-one
4-fluoro-biphenyl
2-hydroxy-5-(4'-fluorophenyl)benzoic acid
CAS:156573-09-0
CAS:143418-49-9
CAS:4009-98-7
CAS:768-35-4