Your Location:Home >Products >Chemical Reagents >768-35-4

Product Details
|
Chemical Properties |
white to light yellow crystal powder |
|
Uses |
suzuki reaction |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C26H4F20P2/c27-3-7(31)15(39)23(16(40)8(3)32)47(24-17(41)9(33)4(28)10(34)18(24)42)1-2-48(25-19(43)11(35)5(29)12(36)20(25)44)26-21(45)13(37)6(30)14(38)22(26)46/h1-2H2
Thienopyrimidine-based allosteric inhibi...
Organozinc pivalates, a recently develop...
This letter presents synthesis and struc...
The process development for the synthesi...
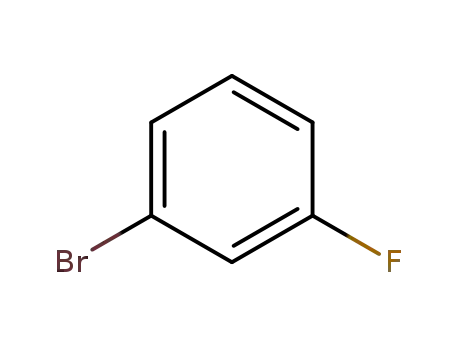
3-fluorobromobenzene

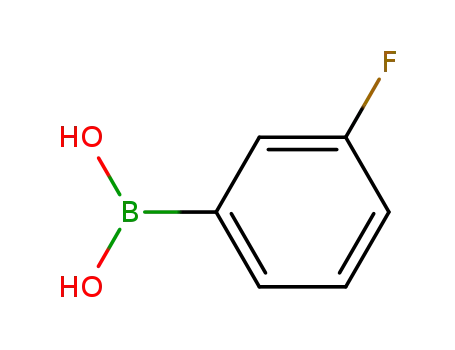
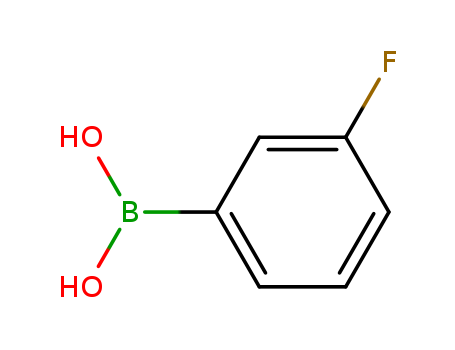
3-fluorophenylboronic acid
| Conditions | Yield |
|---|---|
|
3-fluorobromobenzene;
With
diisobutylaluminium hydride; magnesium; lithium chloride;
In
tetrahydrofuran; toluene;
at 20 ℃;
for 15h;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; toluene;
at 0 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran; toluene;
at 0 ℃;
Inert atmosphere;
|
83% |
|
3-fluorobromobenzene;
With
n-butyllithium;
In
tetrahydrofuran; toluene;
at -78 ℃;
for 1.5h;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; toluene;
at -78 - 20 ℃;
for 1h;
Inert atmosphere;
|
70% |
|
3-fluorobromobenzene;
With
magnesium;
In
diethyl ether;
With
Trimethyl borate;
In
diethyl ether;
at 3 ℃;
for 3h;
With
hydrogenchloride;
In
diethyl ether;
|
|
|
3-fluorobromobenzene;
With
magnesium;
In
tetrahydrofuran;
With
Trimethyl borate;
With
hydrogenchloride;
Further stages.;
|
|
|
3-fluorobromobenzene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 3h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
With
hydrogenchloride;
In
tetrahydrofuran; water;
for 1h;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran / 1 h / -78 °C / Inert atmosphere; Schlenk technique
1.2: -78 - 20 °C / Inert atmosphere; Schlenk technique
2.1: hydrogenchloride / water / 20 °C / Inert atmosphere; Schlenk technique
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran; water;
|
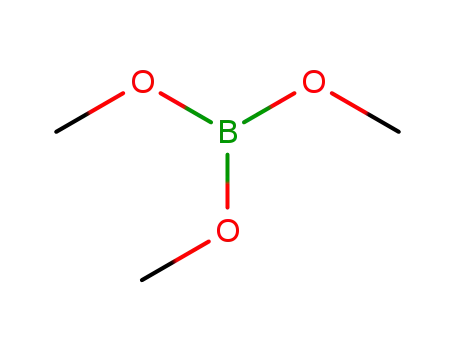
Trimethyl borate


3-fluorobromobenzene


3-fluorophenylboronic acid
| Conditions | Yield |
|---|---|
|
With
n-butyllithium;
In
tetrahydrofuran;
N2; BuLi dropping into org. compd. soln. at -78°C, mixt. keeping 3 h, B-compd. soln. addn. dropwise at -78°C, mixt. allowing to warm to room temp. overnight , stirring 1 h with 10% HCl, extn. (Et2O); org. extracts washing (water), drying, solvent vac. removal;
|
|
|
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
|
boric acid tributyl ester

3-fluorobromobenzene
3-chlorofluorobenzene

Trimethyl borate
tris(3-fluoro phenyl)boroxine
1-(benzyloxy)-4-ethyl-2-(3-fluorophenyl)-5-<(6-methyl-6-cyanoheptyl)oxy>benzene
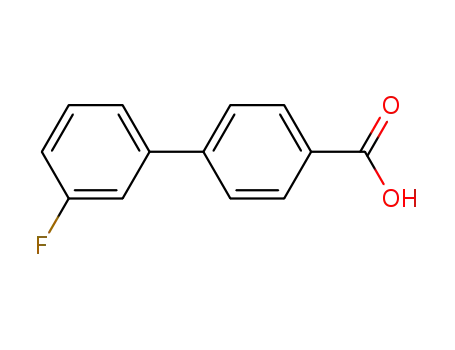
3′-fluoro-(1,1′-biphenyl)-4-carboxylic acid
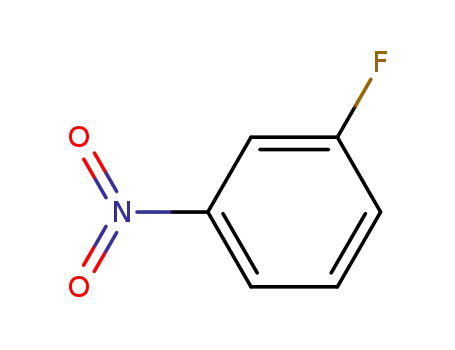
3-fluoro-1-nitrobenzene
CAS:156573-09-0
CAS:1765-93-1
CAS:143418-49-9
CAS:637-88-7