Your Location:Home >Products >Organic Chemistry >143418-49-9
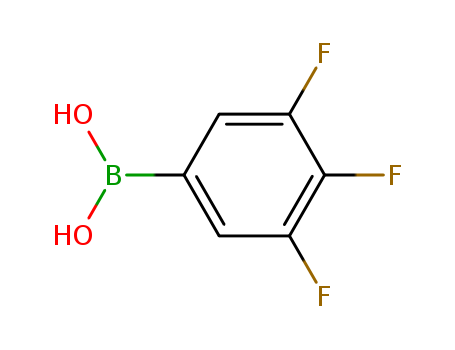

Product Details
|
Uses |
Reactant involved in: ??Preparation of phenylboronic catechol esters and determination of Lewis acidity ??Synthesis of benzopyranone derivatives as GABAA receptor modulators ??Synthesis of multisubstituted olefins and conjugate dienes ??Suzuki cross-coupling reactions ??Preparation of fluorinated aromatic poly(ether-amide)s |
|
Chemical Properties |
Tan powder |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C6H4BF3O2/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,11-12H
The generally accepted monoacyloxyboron ...
The invention provides a preparation met...
The invention relates to a method for th...
The invention discloses an application m...
The invention discloses a 3 ', 4', 5 '- ...
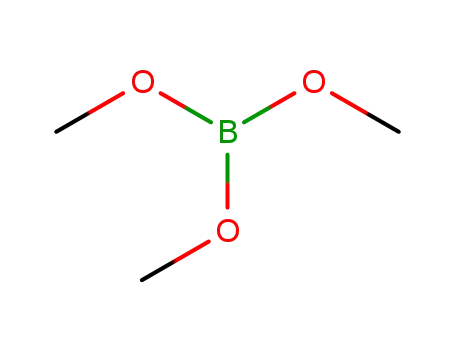
Trimethyl borate

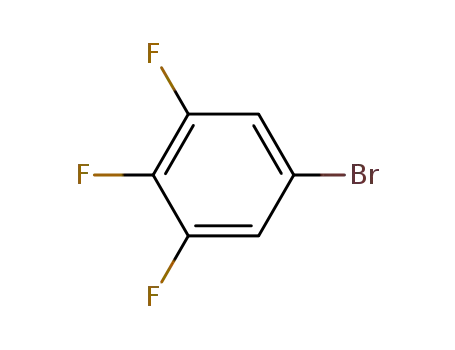
3,4,5-trifluoro-1-bromobenzene

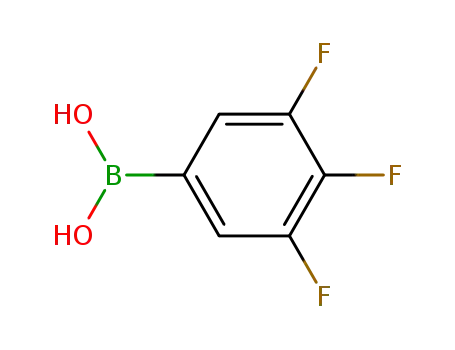
3,4,5-trifluorophenylboronic acid
| Conditions | Yield |
|---|---|
|
3,4,5-trifluoro-1-bromobenzene;
With
magnesium; ethylene dibromide;
In
tetrahydrofuran;
at 5 - 20 ℃;
for 2h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -30 - -27 ℃;
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 20 ℃;
for 4h;
Temperature;
|
82% |
|
3,4,5-trifluoro-1-bromobenzene;
With
magnesium;
In
tetrahydrofuran; ethylene dibromide;
at 5 - 25 ℃;
for 2h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran; ethylene dibromide;
at -30 - -27 ℃;
for 2h;
Inert atmosphere;
With
hydrogenchloride;
In
tetrahydrofuran; water; ethylene dibromide;
at 20 ℃;
for 4h;
Temperature;
Inert atmosphere;
|
81% |
|
With
magnesium;
In
diethyl ether;
Grignard-comp. prepd. from Br-compd. and Mg in ether at 0 °C was added dropwise to 1.5 equiv. of pre-cooled (0 °C) soln. of B(OMe)3 in ether, mixt. was maintained at 0-10 °C for 1 h and allowed to warm up to 20 °C; pouring into 10 % HCl, org. layer was sepd., aq. layer was twice extd. with ether, extracts were combined, dried with MgSO4, evapd. under vac., end-product was contaminated with dehydration product;
|
|
|
3,4,5-trifluoro-1-bromobenzene;
With
magnesium;
In
tetrahydrofuran;
at 25 - 35 ℃;
for 7h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at -5 - 25 ℃;
for 4.5h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
at 25 - 50 ℃;
for 1h;
|
|
|
3,4,5-trifluoro-1-bromobenzene;
With
magnesium;
In
2-methyltetrahydrofuran;
at 20 - 80 ℃;
for 4h;
Inert atmosphere;
Trimethyl borate;
In
2-methyltetrahydrofuran;
at -5 - 20 ℃;
for 2.5h;
With
hydrogenchloride;
In
2-methyltetrahydrofuran; water;
at 20 - 50 ℃;
for 1h;
|

Trimethyl borate

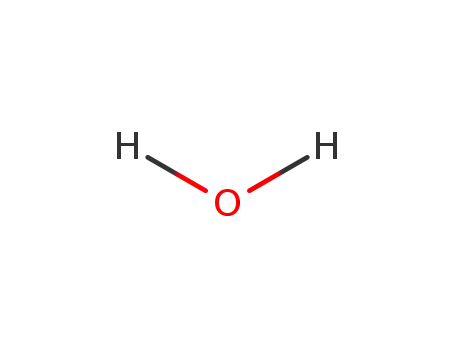
water

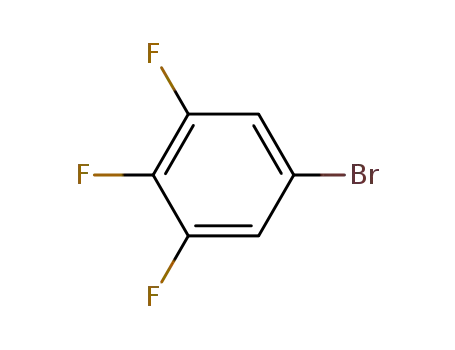
3,4,5-trifluoro-1-bromobenzene


3,4,5-trifluorophenylboronic acid
| Conditions | Yield |
|---|---|
|
3,4,5-trifluoro-1-bromobenzene;
With
iodine; magnesium;
In
2-methyltetrahydrofuran;
at 40 - 50 ℃;
Trimethyl borate;
In
2-methyltetrahydrofuran;
at 10 - 20 ℃;
for 8h;
water;
With
hydrogenchloride;
In
2-methyltetrahydrofuran;
Temperature;
|
78% |
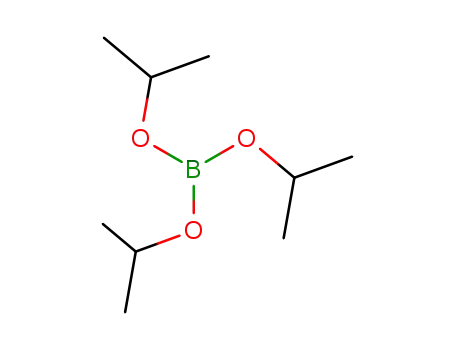
Triisopropyl borate
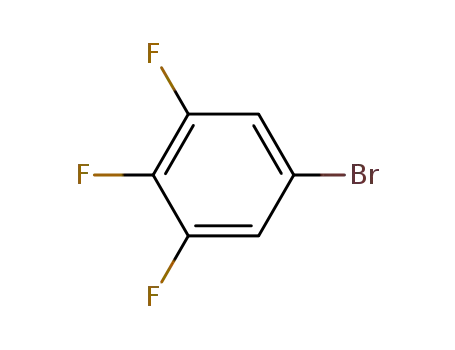
3,4,5-trifluoro-1-bromobenzene

Trimethyl borate
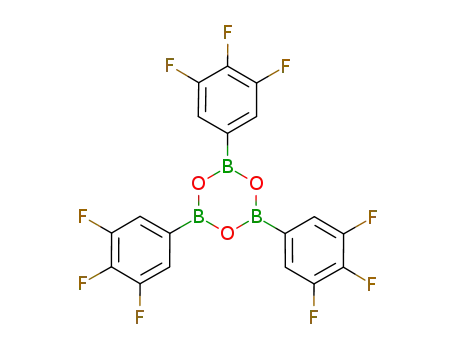
tris(3,4,5-trifluorophenyl)boroxine
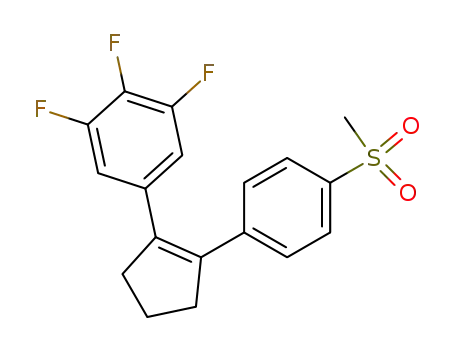
1-[2-(3,4,5-trifluorophenyl)cyclopenten-1-yl]-4-(methylsulfonyl)benzene
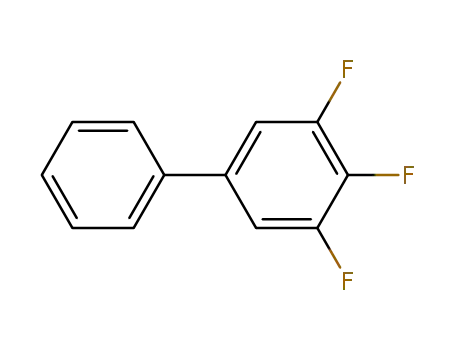
3,4,5-trifluoro-1-1’-biphenyl
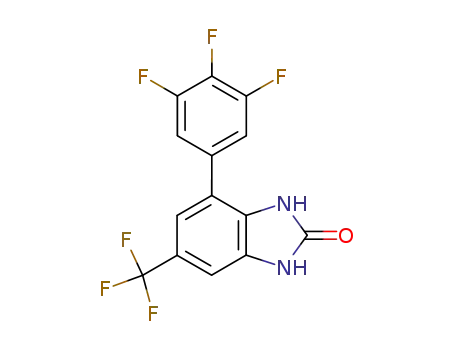
6-(trifluoromethyl)-4-(3,4,5-trifluorophenyl)-1,3-dihydro-2H-benzimidazol-2-one
CAS:156573-09-0
CAS:1765-93-1
CAS:4009-98-7
CAS:768-35-4