Your Location:Home >Products >Flavors and fragrances >629-19-6

Product Details
|
Chemical Properties |
CLEAR COLURLESS TO PALE YELLOW LIQUID |
|
Occurrence |
Reported found in cabbage, onion, garlic, shallot, roasted onion, raw leek, heated leek, chive, nobiru, caucas, Welsh onion, scallion, grilled and roasted beef and roasted peanut. |
|
Preparation |
By boiling propyl bromide and Na2S2 in propyl alcohol; from iodine and n-propyl mercaptan; from propyl iodide and sodium thiosulfate by way of sodium propyl thiosulfate, followed by heating. |
|
Taste threshold values |
Taste characteristics at 10 ppm: alliaceous, sulfurous, green, vegetative and asefetida nuances. |
|
General Description |
Dipropyl disulfide (DPDS) was an efficient scavenger of reactive oxygen species (ROS) at a lower concentration in both HL-60 and HepG2 cells. It prevents the oxidative DNA damage caused by N-nitrosopiperidine (NPIP) and N-nitrosodibutylamine (NDBA). |
|
Who Evaluation |
Evaluation year: 2007 |
|
EXPOSURE ROUTES |
inhalation, ingestion, skin and/or eye contact |
|
FIRST AID |
(See procedures) Eye:Irrigate immediately Skin:Soap wash immediately Breathing:Respiratory support Swallow:Medical attention immediately |
InChI:InChI=1/C6H14S2/c1-3-5-7-8-6-4-2/h3-6H2,1-2H3
Reaction of dichlorobenzaldazine (2) wit...
Reaction of Tb(III) and two bridging lig...
The efficient and straightforward synthe...
A number of substituted o-aminophenols h...
A copper-catalyzed direct C-H chalcogena...
Various embodiments disclosed relate to ...
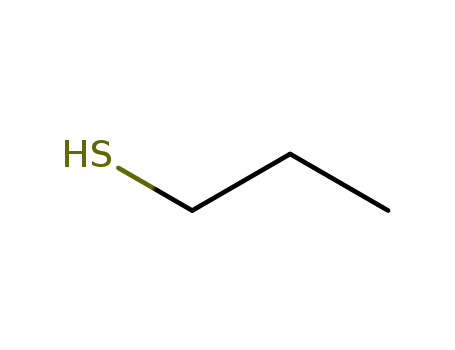
1-thiopropane

para-nitrobenzenethiol

di(p-nitrophenyl) disulfide

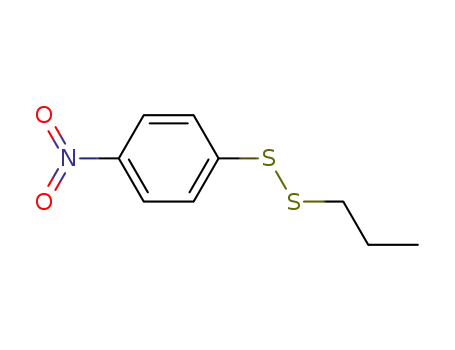
1-(4-nitrophenyl)?2-propyldisulfane

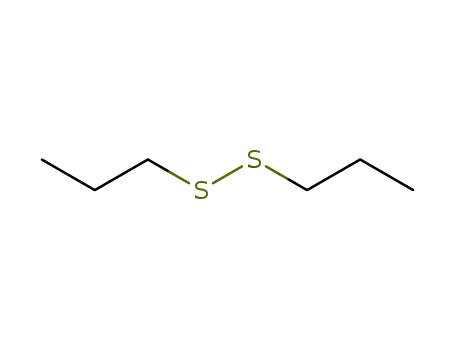
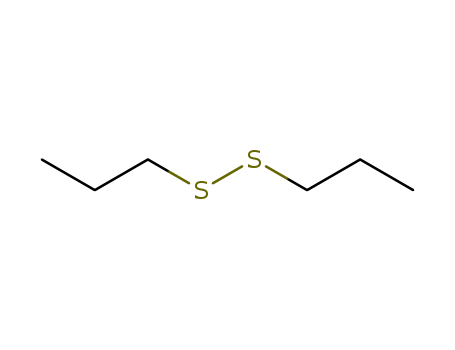
Dipropyl disulfide
| Conditions | Yield |
|---|---|
|
With 4,6-di-tert-butyl-N-(tert-butyl)-o-aminophenol; In acetonitrile; Electrolysis;
|
84% |

1-thiopropane

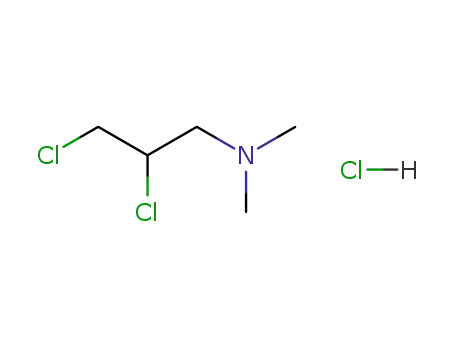
1-(N,N-dimethylamino)-2,3-dichloropropane hydrochloride

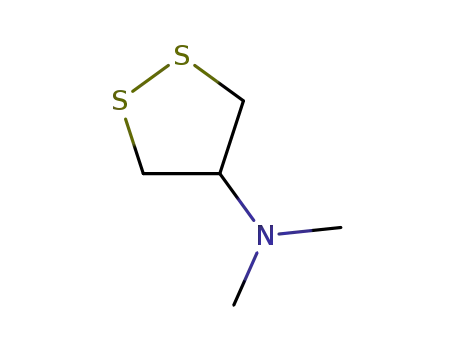
nereistoxin

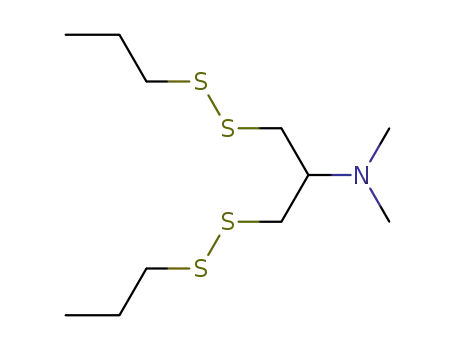
Dimethyl-(2-propyldisulfanyl-1-propyldisulfanylmethyl-ethyl)-amine

![[2-(2-Dimethylamino-3-propyldisulfanyl-propyldisulfanyl)-1-propyldisulfanylmethyl-ethyl]-dimethyl-amine](/upload/2023/8/ad10a7fc-2268-4ec0-aeb7-5ab315b7fb8c.png)
[2-(2-Dimethylamino-3-propyldisulfanyl-propyldisulfanyl)-1-propyldisulfanylmethyl-ethyl]-dimethyl-amine


Dipropyl disulfide
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; sodium thiosulfate; sodium chloride; Yield given. Multistep reaction. Yields of byproduct given; 1) EtOH, H2O, reflux, 30 min., 2) H2O, CHCl3, 3 h;
|
|
|
With sodium hydroxide; sodium thiosulfate; sodium chloride; Yield given. Multistep reaction. Further byproducts given. Yields of byproduct given; 1) EtOH, H2O, reflux, 30 min., 2) H2O, CHCl3, 2.1) 3 h, 2.2) 2-4 h (pH 11);
|
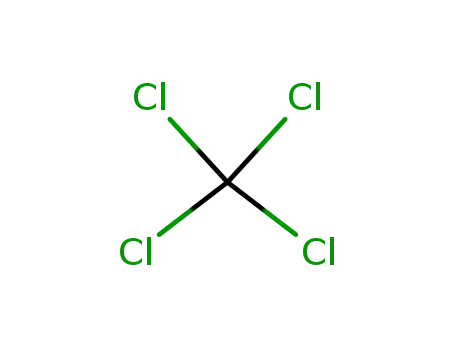
tetrachloromethane

1-thiopropane
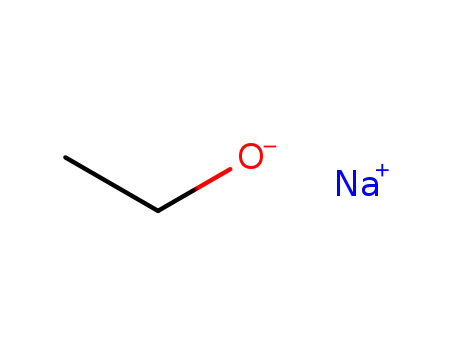
sodium ethanolate
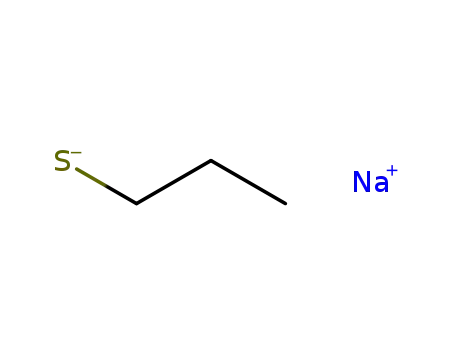
sodium 1-propanethiolate
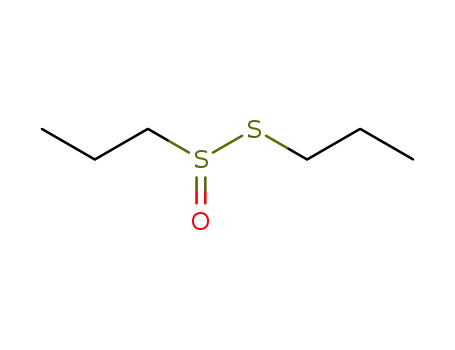
propyl propylthiosulfinate
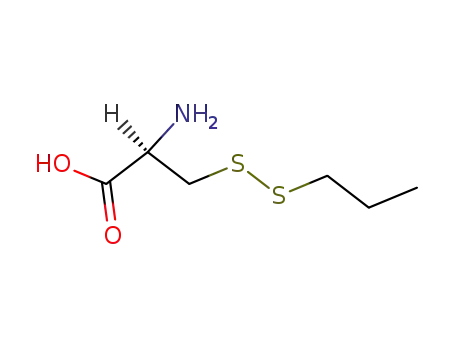
S-propylmercapto-L-cysteine
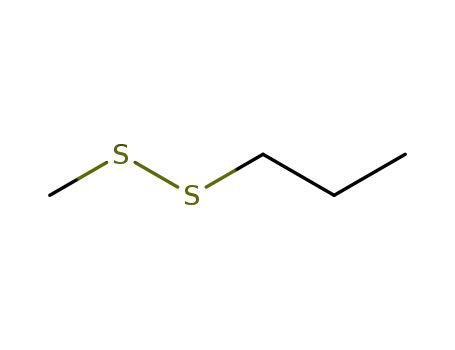
methyl n-propyl disulfide
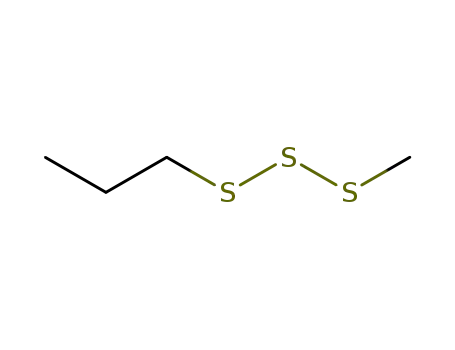
methyl propyl trisulfide
CAS:24295-03-2
CAS:54812-86-1
CAS:22047-25-2
CAS:34413-35-9