Your Location:Home >Products >Flavors and fragrances >24295-03-2
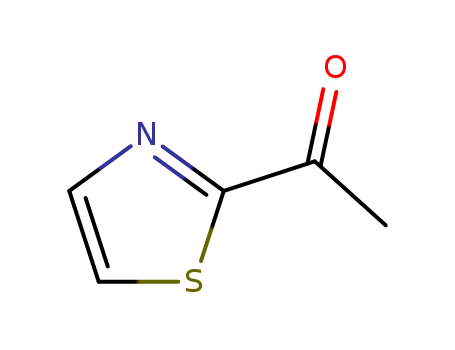

Product Details
|
Chemical Properties |
2-Acetylthiazole is a colorless to pale yellow liquid with green onion, herbal, grassy, hazelnuts, roasted meat, caramel, corn, and butter odor. Natural products are found in beef broth. Slightly soluble with water, soluble in most organic solvents. It is used in foods as antioxidant, as appearance control agent, and as flavoring ingredient. |
|
Occurrence |
2-Acetylthiazole is naturally found in raw asparagus, cooked asparagus, kohlrabi, cooked or boiled potatoes, turkey (roasted), raw chicken, boiled and cooked beef, grilled and roasted beef, pork liver, beer, Finnish whiskey, heated beans, other varieties of mushroom, rice bran, maize, and other natural sources. |
|
Uses |
2-Acetylthiazole can be used as a flavoring agent in food industries. It may be used as a food additive in the preparation of ′fragrant′ rice. It also was used in the preparation of triazolothiazoles, chiral alcohols and in aldol condensation reactions. |
|
Preparation |
2-Acetylthiazole is formed by oxidation of the corresponding carbinol using dichromate. |
|
Aroma threshold values |
Detection at 4 ppb |
|
Taste threshold values |
Taste characteristics at 30 ppm: corn chip with slightly musty background |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 53, p. 1748, 1988 DOI: 10.1021/jo00243a029 |
|
General Description |
2-Acetylthiazole is a volatile flavoring substance generally formed by the Maillard reaction between an amino acid and carbonyl compounds. It is reported to occur in canned sweet corn products, cooked pine mushroom, cooked asparagus and roasted beef. |
|
Purification Methods |
Check NMR spectrum; if it is not too bad, distil it through an efficient column in a vacuum. The oxime sublimes at 140-145o, m 159o, and when crystallised from H2O has m 163-165.5o. [Erlenmeyer et al. Helv Chim Acta 31 1142 1948, Waisvisz et al. J Am Chem Soc 79 4524 1957, Menasseé et al. Helv Chim Acta 40 554 1957, Beilstein 27 IV 2617.] |
|
Who Evaluation |
Evaluation year: 2002 |
InChI:InChI=1/C5H5NOS/c1-4(7)5-6-2-3-8-5/h2-3H,1H3
The role of amino acids and α-dicarbonyl...
Herein, a new procedure for the carbonyl...
The invention relates to a synthetic met...
The sulphur aroma compounds produced fro...
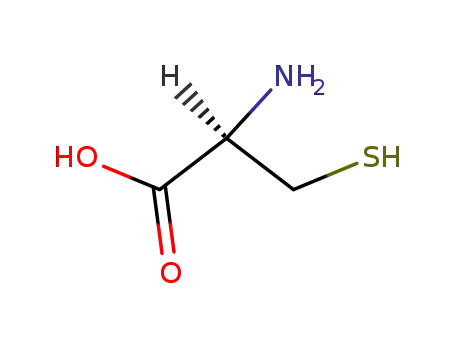
L-Cysteine

[1-13C]ascorbic acid

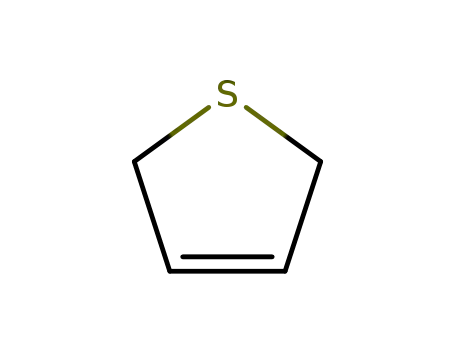
2,5-dihydro-thiophene

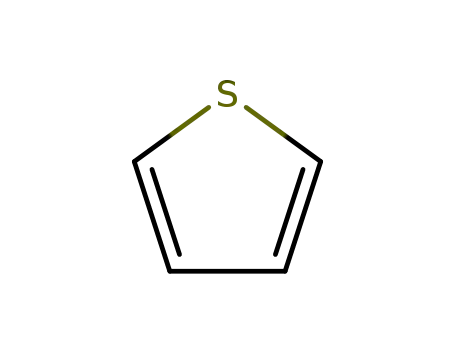
thiophene

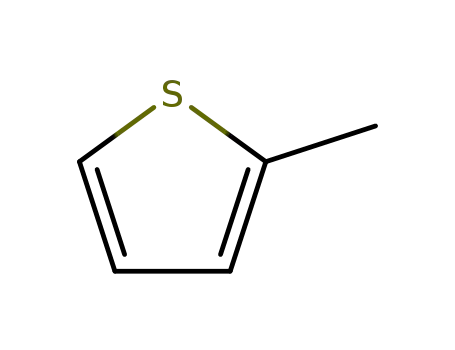
2-Methylthiophene

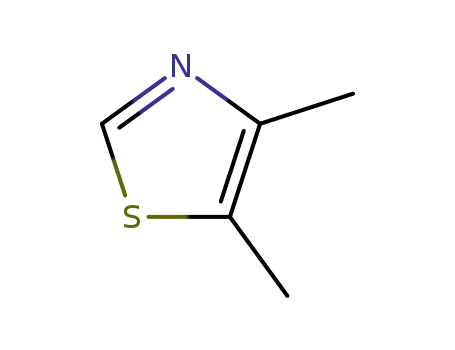
4,5-Dimethylthiazole

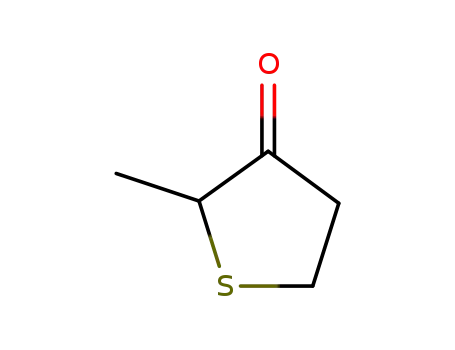
4,5-Dihydro-2-methylthiophen-3-(2H)-on

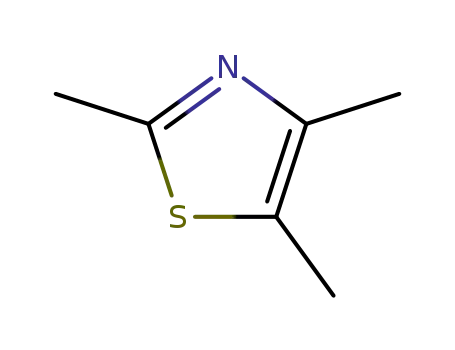
2,4,5-trimethylthiazole

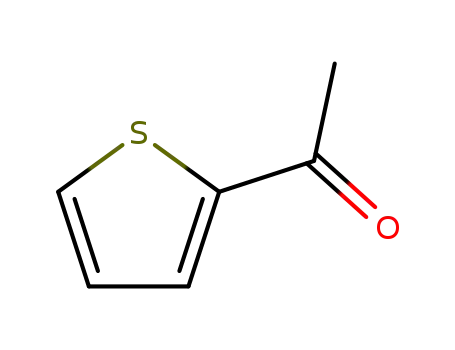
2-Acetylthiophene

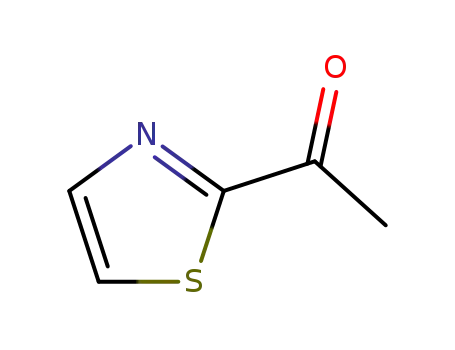
2-Acetylthiazole

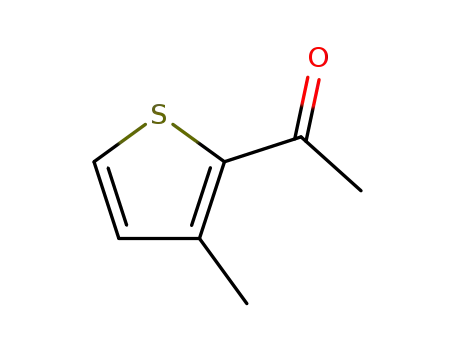
2-acetyl-3-methylthiophene

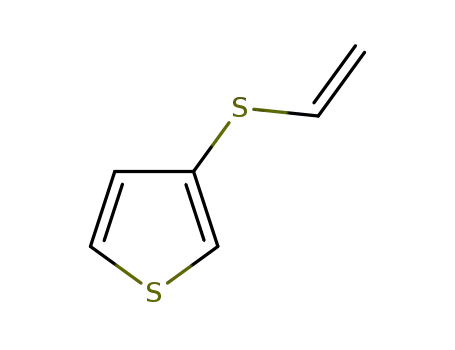
3-(vinylthio)thiophene
| Conditions | Yield |
|---|---|
|
at 140 ℃; for 2h; pH=8; aq. phosphate buffer; Sealed vessel;
|

L-Cysteine

ascorbic acid


2,5-dihydro-thiophene


thiophene


2-Methylthiophene


4,5-Dimethylthiazole


4,5-Dihydro-2-methylthiophen-3-(2H)-on


2,4,5-trimethylthiazole


2-Acetylthiophene


2-Acetylthiazole


2-acetyl-3-methylthiophene


3-(vinylthio)thiophene
| Conditions | Yield |
|---|---|
|
at 140 ℃; for 2h; pH=8; aq. phosphate buffer; Sealed vessel;
|
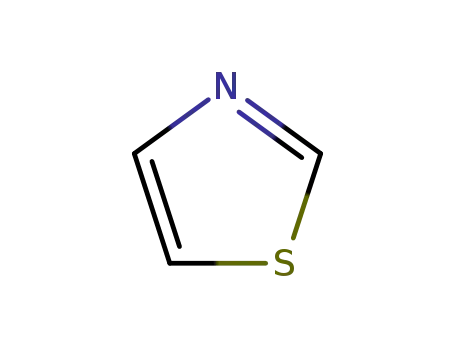
1,3-thiazole
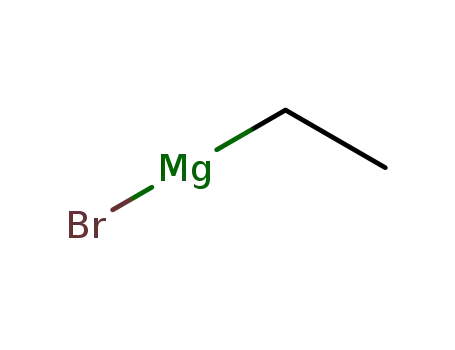
ethylmagnesium bromide
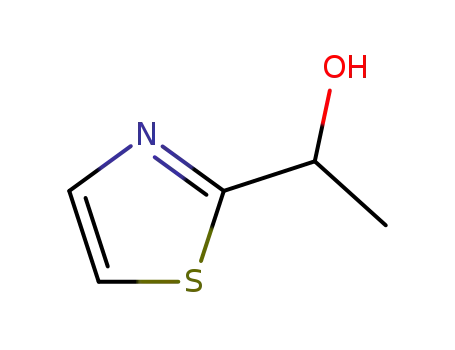
(±)-1-(thiazol-2-yl)ethanol
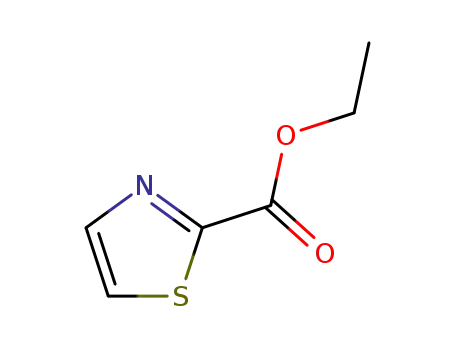
thiazole-2-carboxylic acid ethyl ester
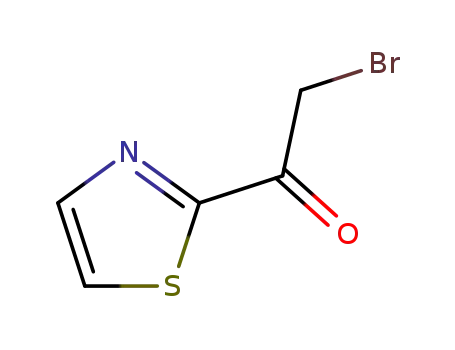
2-bromo-1-thiazol-2-yl-ethanone
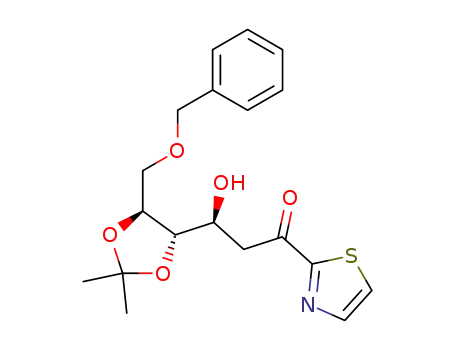
(3S,4S,5S)-6-O-benzyl-4,5-O-isopropyliden-3,4,5,6-tetrahydroxy-1-(2-thiazolyl)-1-hexanone
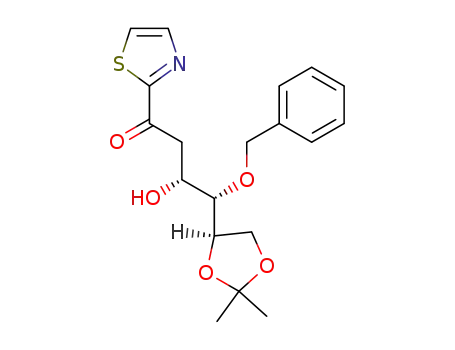
(3R,4S)-4-Benzyloxy-4-((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-3-hydroxy-1-thiazol-2-yl-butan-1-one
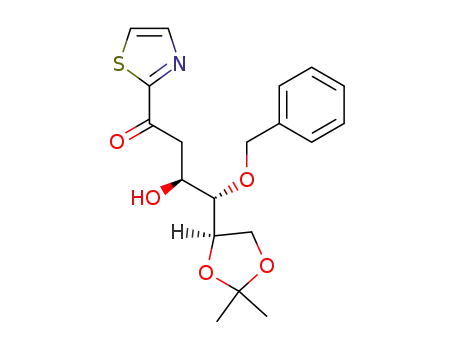
(3S,4R,5R)-4-O-Benzyl-5,6-O-isopropylidene-3,4,5,6-tetrahydroxy-1-(2-thiazolyl)-1-hexanone
CAS:137-00-8
CAS:2495-39-8
CAS:22047-25-2
CAS:629-19-6