Your Location:Home >Products >Chemical Reagents >637-88-7
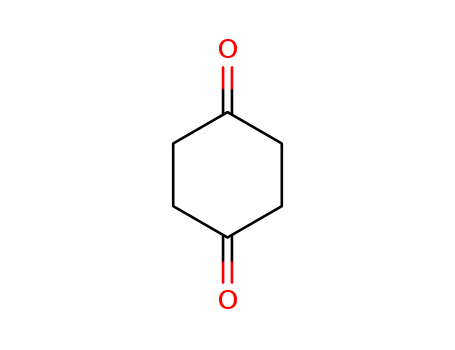

Product Details
|
Chemical Properties |
tan or yellow crystalline powder |
|
Uses |
1,4-Cyclohexanedione is used in the preparation of 1,4 benzoquinone and bromoorganics. It is also used to study the influence of visible light on the bromate-1,4-cyclohexanedione-ferroin oscillating reaction. It plays a vital role in pharmaceuticals, plant growth regulator and as a conducting material. |
|
Definition |
ChEBI: 1,4-Cyclohexanedione is a cyclohexanedione with oxo substituents at positions 1 and 4. |
|
Preparation |
Synthesis of 1,4-cyclohexanedione: put diethyl succinylsuccinate into a flask, add a mixture of concentrated sulfuric acid, water and ethanol, reflux in oil solution for 5 days, cool, and neutralize to pH with ammonia water = 8; then extract 4 times with chloroform, and recover the chloroform to obtain the crude product; then the crude product is subjected to vacuum distillation, and the distillate is poured into cold petroleum ether, filtered, and air-dried to obtain 1,4-cyclohexanedi Ketone Products. |
|
Synthesis Reference(s) |
Synthesis, p. 165, 1981 DOI: 10.1055/s-1981-29377 |
|
General Description |
1,4-Cyclohexanedione(CHD) undergoes uncatalyzed oscillatory reactions during oxidation by acidic bromate in nitric acid and sulphuric acid solution. It reacts with acidic bromate to form 1,4-dihydroxybenzene which on further oxidation and bromination yields 1,4-benzoquinone and bromoorganics. |
|
Pharmacology |
The cyclohexanedione (CHD) herbicides inhibit fatty acid synthesis in plants by interfering with the activity of the enzyme Acetyl-Coenzyme A Carboxylase (ACCase). ACCase-inhibiting herbicides provide excellent control of grass weeds in dicotyledonous and some grass crops. A less-sensitive ACCase mediates the intrinsic resistance of dicotyledonous plants to the AOPP and CHD herbicides (34,35). Although grasses are target species of this group of herbicides, not all are equally affected, and sensitivity differences can occur between varieties or even within a genus. |
InChI:InChI=1/C6H8O2/c7-5-1-2-6(8)4-3-5/h1-4H2
-
The detection of H2, HD, and D2 by gas-p...
-
We have reported an aerobic oxidation of...
Catalytic oxidation reaction using green...
The invention discloses a hydrolysis pro...
The reductive activation of molecular ox...
cyclohexa-1,4-diene

cyclohexenone

cyclohex-3-enone

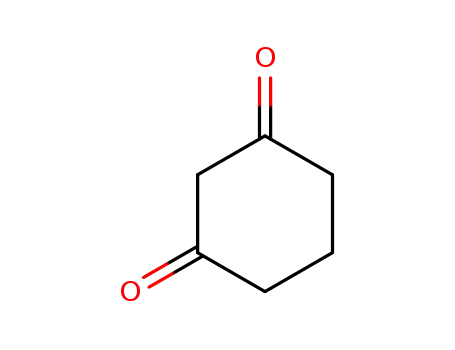
1,3-cylohexanedione

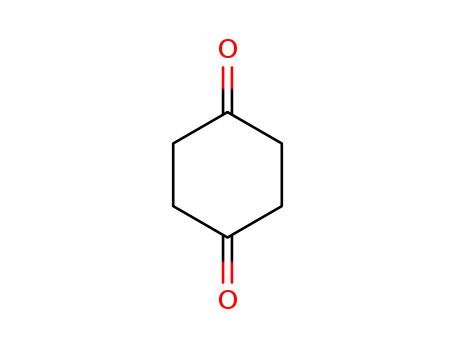
1,4-Cyclohexanedione
| Conditions | Yield |
|---|---|
|
With
dinitrogen monoxide;
at 250 ℃;
for 5h;
pressure;
|
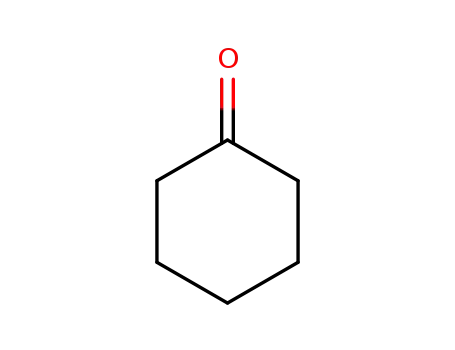
cyclohexanone


cyclohexenone


cyclohex-3-enone

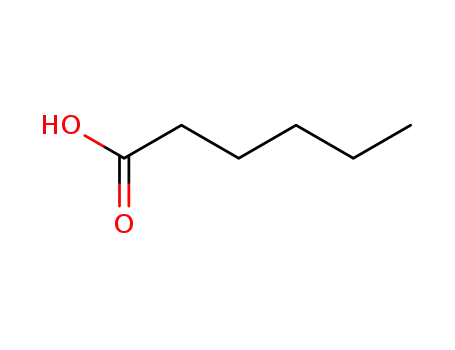
hexanoic acid


1,4-Cyclohexanedione
| Conditions | Yield |
|---|---|
|
With
sodium persulfate; iron(II) sulfate;
In
water;
Yield given. Yields of byproduct given;
|
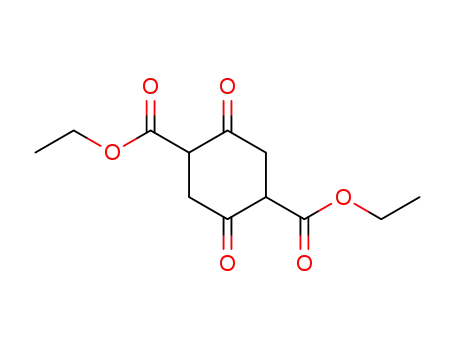
diethyl 1,4-cyclohexanedione-2,5-dicarboxylate
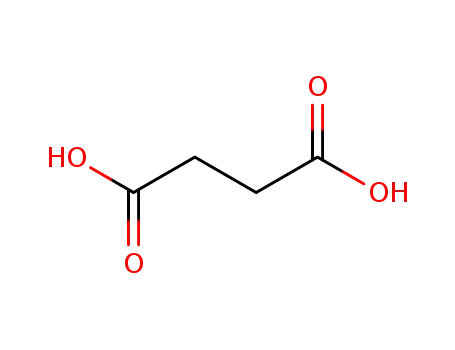
succinic acid
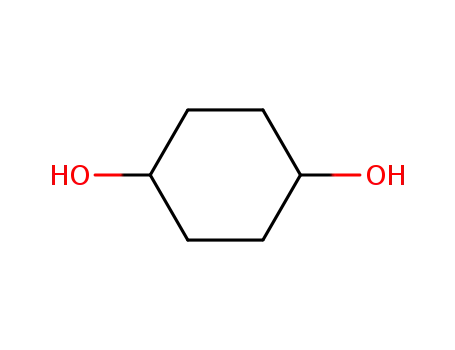
1,4-Cyclohexanediol
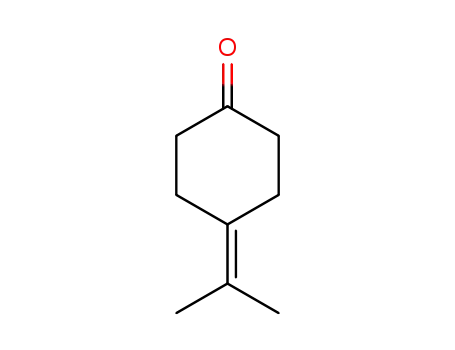
4-(propan-2-ylidene)cyclohexan-1-one
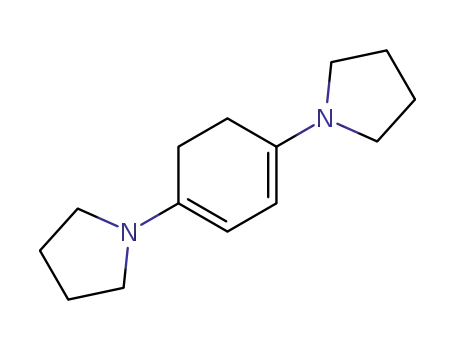
1,4-dipyrrolidino-cyclohexa-1,3-diene
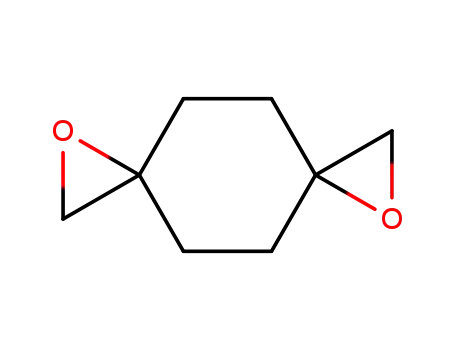
1,7-dioxa-dispiro[2.2.2.2]decane
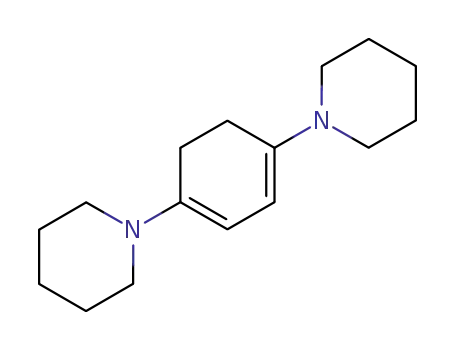
1,4-dipiperidino-cyclohexa-1,3-diene
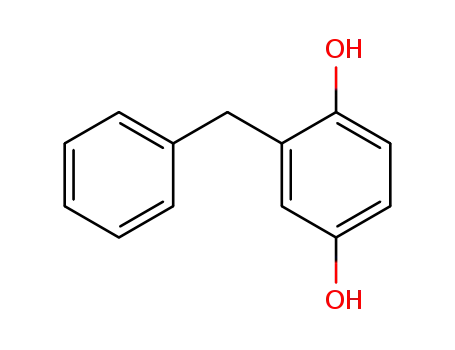
benzylhydroquinone
CAS:22047-25-2
CAS:24295-03-2
CAS:768-35-4
CAS:22803-05-0