Your Location:Home >Products >Chemical Reagents >17455-13-9
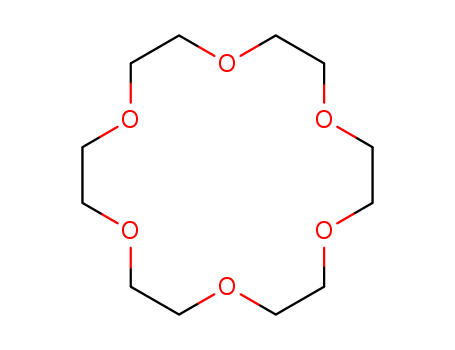

Product Details
|
Chemical Properties |
slightly yellow solid |
|
Uses |
A useful phase transfer catalyst. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 39, p. 2445, 1974 DOI: 10.1021/jo00930a037 |
|
General Description |
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes. |
|
Purification Methods |
Recrystallise it from acetonitrile and dry it in a vacuum. Purify it also by precipitating the 18-crown-6/nitromethane 1:2 complex with Et2O/nitromethane (10:1 mixture). The complex is decomposed in vacuum whereby 18-crown-6 distils off under the reduced pressure. [Beilstein 19/12 V 601.] |
InChI:InChI=1/C12H24O6/c1-2-14-5-6-16-9-10-18-12-11-17-8-7-15-4-3-13-1/h1-12H2
(Chemical Equation Presented) 4-tert-But...
Solution thermodynamic data and solid st...
One-pot syntheses of hexa- and octaethyl...
-
-
DNMR studies show that for complexation ...
Stability constants and thermodynamic va...
A series of crown ethers were synthesize...
Lithium-7 NMR studies have been carried ...
A non-ionic cryptand-22 surfactant consi...
Through the reaction of polyethylene gly...
Several trinuclear titanium complexes be...
We report a new synthetic approach to la...
The preparation of seven new macrocyclic...
Crown lactones may be reduced to crown e...
18-crown-6 ether (18C6) complexes with t...
A new facile method of synthesizing crow...
The cyclotetrasilane [(i-Pr)2Si]4 reacts...
The reaction between and LiBu, LiBu-1,4...
The preparation 18 - comprises the steps...
The invention discloses a preparation me...
The invention discloses a 18 - crown eth...
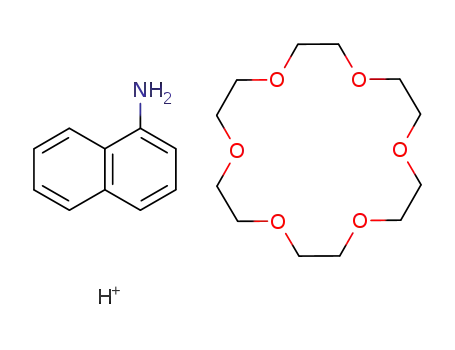
18-crown-6:1-napthylammonium ion 1:1 complex

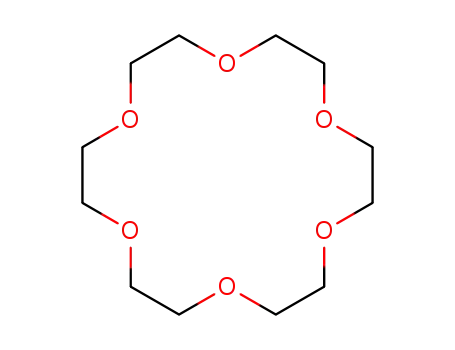
18-crown-6 ether

1-amino-naphthalene
| Conditions | Yield |
|---|---|
|
In
methanol; water;
at 26.9 ℃;
Equilibrium constant;
Thermodynamic data;
ΔG300, ΔH, ΔS;
|
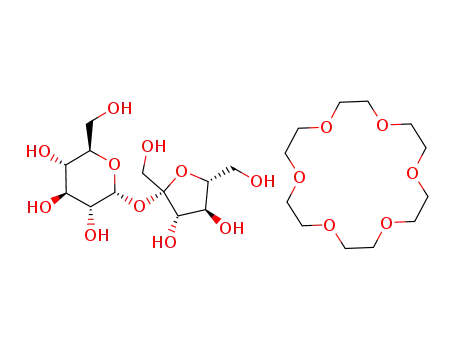
(2R,3R,4S,5S,6R)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis-hydroxymethyl-tetrahydro-furan-2-yloxy)-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol; compound with 1,4,7,10,13,16-hexaoxa-cyclooctadecane


18-crown-6 ether

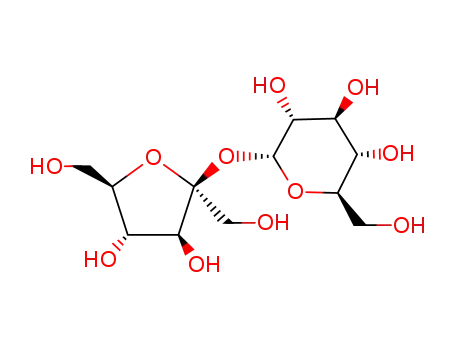
Sucrose
| Conditions | Yield |
|---|---|
|
In
water;
at 25 ℃;
Equilibrium constant;
|
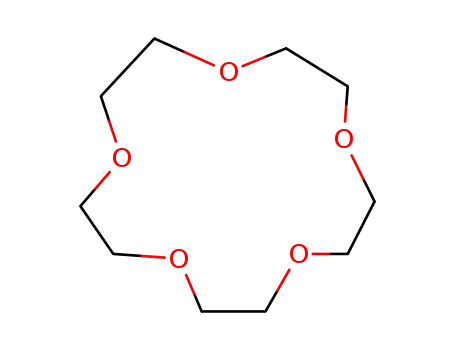
15-crown-5
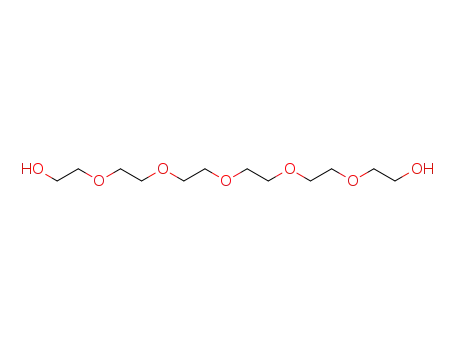
pentaethylene glycol
1,4,7,10,13,16,10-heptaoxacyclohenicosane
(18-crown-6)potassium
2-hydroxymethyl-18-crown-6
1,4,7,10,13,16-Hexaoxa-cyclooctadecane; compound with 4-amino-benzoic acid propyl ester
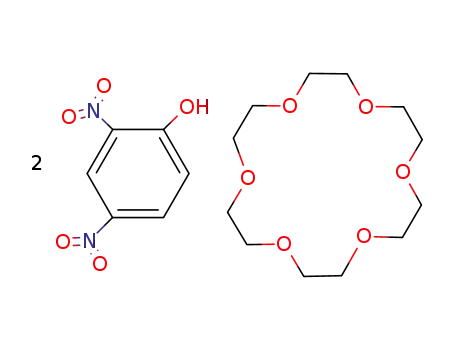
1,4,7,10,13,16-Hexaoxa-cyclooctadecane; compound with 2,4-dinitro-phenol
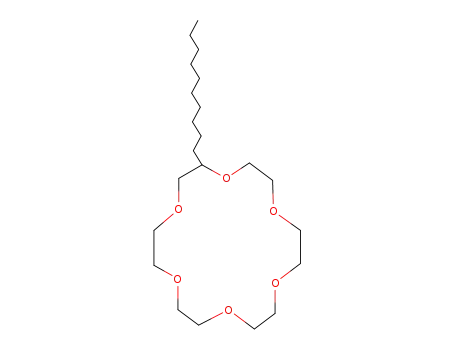
n-Decyl-18-crown-6
CAS:22047-25-2
CAS:24295-03-2
CAS:56-93-9
CAS:1667-10-3